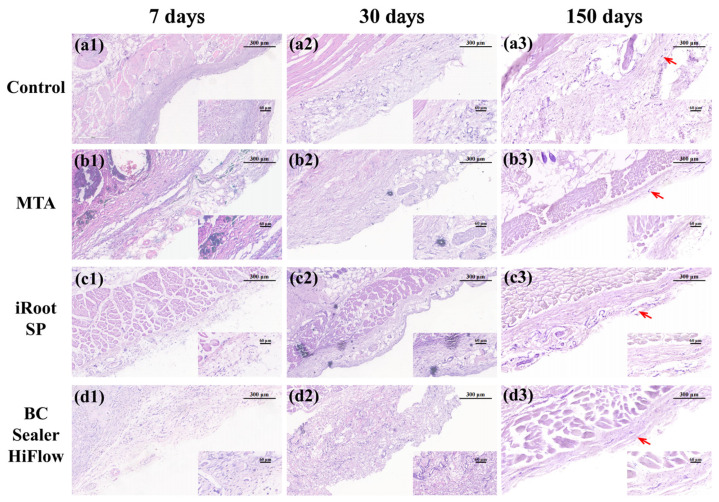
Figure 3.
In vivo tissue response to three biomaterials. (a1–d1) H&E staining of subcutaneous connective tissues in the control, MTA, iRoot SP and BC Sealer HiFlow groups at 7 days. All the groups exhibited mostly moderate-to-severe inflammation. MTA exposure resulted in more visual fields with severe inflammation, whereas iRoot SP followed and BC Sealer HiFlow resulted in the lowest number of fields with severe inflammation. (a2–d2) H&E staining of subcutaneous connective tissues in the control, MTA, iRoot SP and BC Sealer HiFlow groups at 30 days. The control and MTA treatments resulted in mild inflammation. The SP and HiFlow treatments resulted in mostly mild-to-moderate inflammation. (a3–d3) H&E staining of subcutaneous connective tissues in the control, MTA, iRoot SP and BC Sealer HiFlow groups at 150 days. All the groups exhibited fibrous connective tissue capsules (red arrows) with minimal inflammation. Over time, the presence of macrophages and necrotic areas generally declined. (100×, bar, 300 μm; insets show tissue details at 400×, bar, 60 μm).