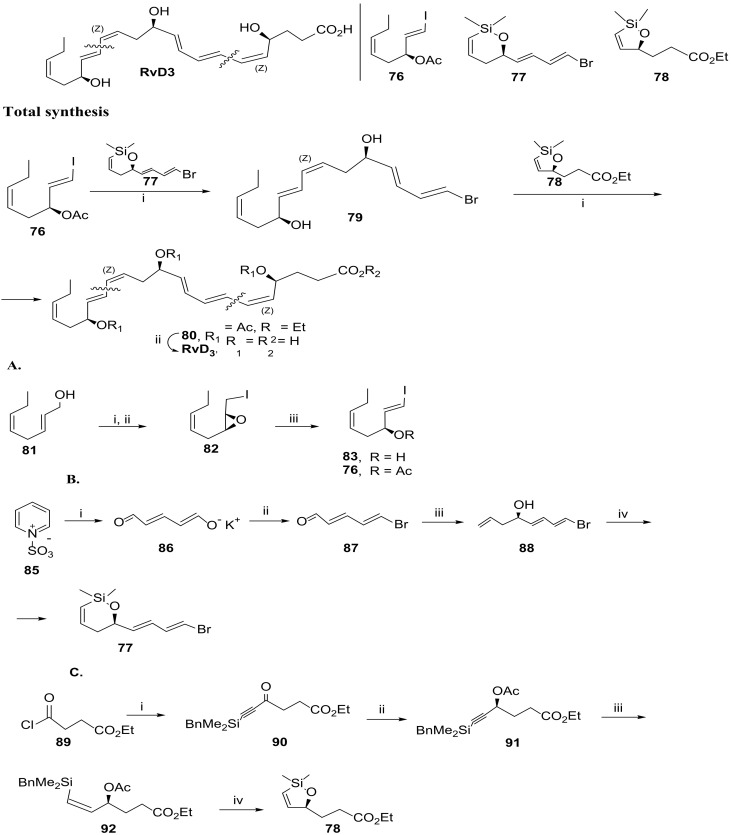
Scheme 13.
Synthesis of RvD3, as reported by Anderson and co-workers [88], and preparation of the building blocks 76 (A), 77 (B), and 78 (C). Reagents and conditions: Total synthesis: i. 1. Pd(dba)2, TBAF, THF, 16 h; 2. Ac2O, py, CH2Cl2, 56% for 79 and 32% for 80, both over the two steps ii. LiOH, THF/H2O (1:1), 99%; (A). Preparation of 76: i. 1. Ti(O-iPr)4, (l)-(+)-DIPT, TBHP, 3 Å MS, CH2Cl2, −20 °C, 75%, 92% ee; 2. I2, PPh3, imidazole, CH2Cl2, 53%; iii. Ac2O, DMAP, Et3N, CH2Cl2, 0 °C to rt, 2 h, 93%; (B). Preparation of 77. i.KOH, 58% [X8]; ii. Ph3PBr2, 50% (isolated yield) [89]; iii. 1.CH2=CHCH2MgCl, THF, 0 °C; 2. Ti(O-iPr)4, (l)-(+)-DIPT, TBHP, 3 Å MS, CH2Cl2, −20 °C, 97%ee, 38% yield over the two steps; iv. 1. CH2 = CHSiMe2Cl, Et3N, CH2Cl2; 2. Schrock catalyst, PhH, 87%; Preparation of 78: i. BnMe2SiC≡CMgBr, 57%; ii. 1. RuCl(S, S)TsDPEN, iPrOH; 2. Ac2O cat., DMAP, Et3N, CH2Cl2, 88%, 97% ee; iii. Pd/CaCO3, quinoline, H2, PhMe-cyclohexene (10:1), 83%; iv. TBAF, THF, quantitative yield.