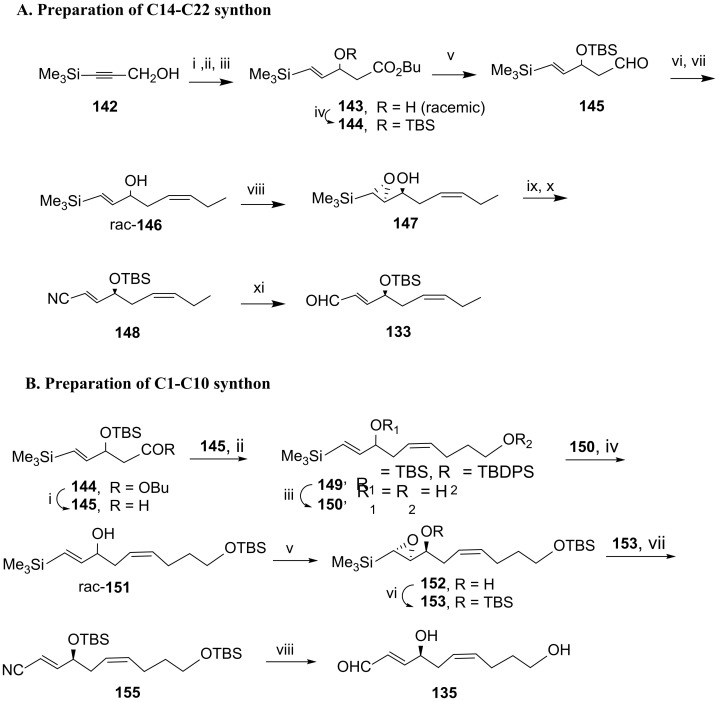
Scheme 20.
Preparation of C14-C22 synthon 133 (A) and C1-C10 synthon 135 (B). Reagents and conditions: (A). i. LiAlH4; ii. PCC; iii. MeCOOBu/LDA, 69% over the three steps; iv. TBSCl, imidazole, 89%; v. DIBAL; vi. PrPPh3Br, NaHMDS, THF, −90 °C to rt, 12 h, vii. TBAF, 74% over the three steps; viii. tBuOOH, Ti(OiPr)4, l-(+)-DIPT, CH2Cl2, −18 °C, 6 h, 47%, 98% ee; ix. TBSOTf, 2,6-lutidine, CH2Cl2, rt, 1.5 h; x. Et2AlCN, toluene, rt, overnight, 79% over the two steps; xi. DIBAL, CH2Cl2, −40 °C to 0 °C, 1 h, 73%. (B). i. DIBAL, −78 °C; ii. TBDPSO(CH2)4PPh3I, NaHMDS, THF, −90 °C to rt, 14 h; iii. TBAF, 91% from 144; iv. TBSCl, CH2Cl2, 0 °C, 3.5 h, 84%; v. tBuOOH, l-(+)-DIPT, Ti(OiPr)4, CH2Cl2, −18 °C, 6 h, 44% and 98% ee; vi. TBSOTf, 2,6-lutidine, CH2Cl2, rt, 3 h; vii. Et2AlCN, toluene, 0 °C, 2 h, 92% over the two steps; viii. DIBAL, CH2Cl2, −70 °C, 1 h, 82%.