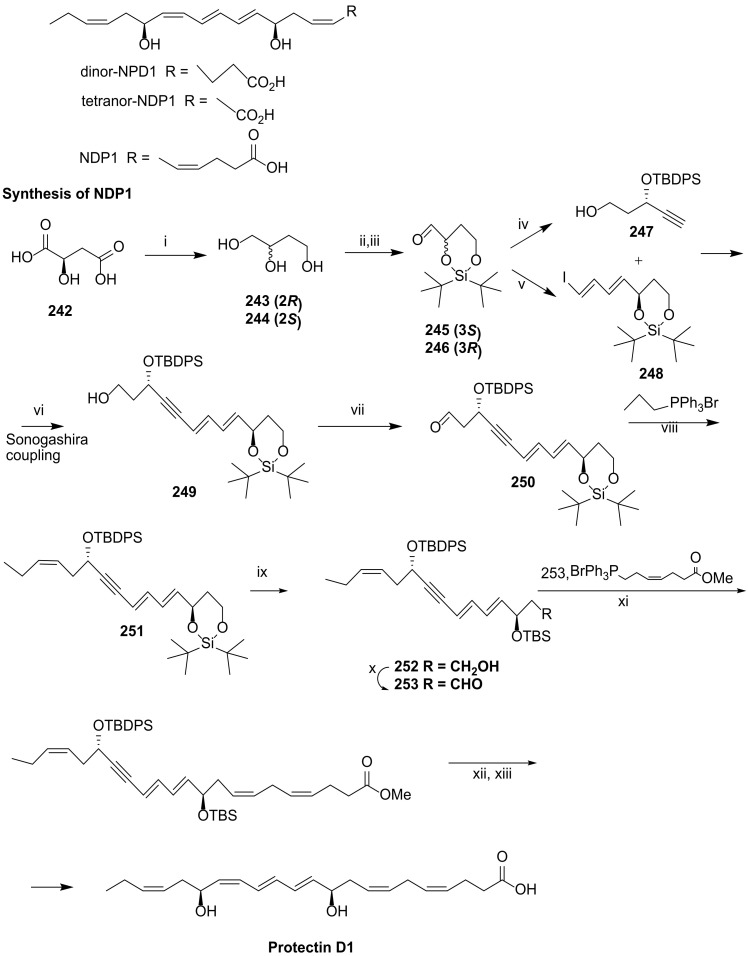
Scheme 30.
Synthesis of protectin D1/NPD1, as reported by Balas and co-workers [108]. Reagents and conditions: i. BH3.SMe2, B(OMe)3,98%; ii. tBu2Si(OTf)2, py, CH2Cl2/DMF (6:4), −30 °C, 2 h; iii. 1. (COCl)2, DMSO; 2. Et3N, CH2Cl2, −78 °C to rt, 1 h, 90% over the two steps; iv. 1. (MeO)2P(=O)-C(=N2)-P(=O)-CH3, K2CO3, MeOH, 0 °C to rt, 3 h; 2. TBAF, THF, 0 °C to rt, 2 h; 4. 1. TESCl, Et3N, CH2Cl2, −25 °C, 15 h; 5. TBDPSCl, TES deprotection, 44% over the 5 steps; v. 1. Ph3P=CHCHO, CH3CN, 30 °C; Me3SiCHN2, LDA, −78 °C to 0 °C, THF, 2.5 h; 3. a. ZrCl2(Cp)2, DIBAL, b. THF, I2; 52% over the three steps; vi. Pd (PPh3)4, CuI, THF, 91%; vii. 1. (COCl)2, DMSO; 2. Et3N, CH2Cl2, −78 °C to rt, 1 h,95%; viii. NaHMDS, THF, −78 °C, 3 h; ix. 1. TBAF, 0 °C to rt, 1 h; 2. TBSCl, imidazole, CH2Cl2, 0 °C to rt, 1 h; 3. PPTS, EtOH, 0 °C to rt, 5.5 h; x. 1. (COCl)2, DMSO 2. Et3N, CH2Cl2, −78 °C to rt, 1 h, 33% over the last five steps; xi Base, THF; xii. Zn(Cu-Ag),26%; xiii. LiOH (1M), MeOH, 97%.