Abstract
Background
Indomethacin is a prostaglandin inhibitor used for the prevention and the treatment of patent ductus arteriosus (PDA). Potential adverse effects of indomethacin use in premature infants include reduction in cerebral, mesenteric and renal blood flow and platelet dysfunction. Administering indomethacin continuously over 36‐hours has been suggested as a safer and more effective option to prevent such adverse effects compared to bolus administration.
Objectives
To compare the efficacy and safety of continuous infusion vs. bolus administration of indomethacin in closing a symptomatic PDA in preterm infants.
Search methods
We searched MEDLINE (via PubMed), CINAHL, EMBASE and CENTRAL (The Cochrane Library) through 2009.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing continuous indomethacin infusion to bolus doses for closure of a symptomatic PDA in preterm infants with a symptomatic PDA diagnosed clinically and/or by echocardiography.
Data collection and analysis
Data collection and analysis were done in accordance with the recommendations of the CNRG.
Main results
Only two small trials comparing continuous vs. bolus indomethacin were eligible. Analysis of these studies showed that there were no statistically significant differences in PDA closure at day 2 (RR 1.57, 95% CI 0.54, 4.60) and at day 5 (RR 2.77, 95% CI 0.33, 23.14). There was no statistical difference between the bolus and continuous groups for the secondary outcomes of reopening of PDA, neonatal mortality, intraventricular hemorrhage and necrotizing enterocolitis. None of the trials reported on outcomes such as requirement for retreatment with indomethacin or surgical ligation, mortality, bronchopulmonary dysplasia, retinopathy of prematurity, neurodevelopmental outcome and isolated intestinal perforation.
The review demonstrated that there was a decrease in cerebral blood flow velocity after bolus injections and that the difference between the bolus and continuous infusion groups remained significant for 12 ‐ 24 hours. Similar decrease in blood flow to the renal and mesenteric circulations following bolus administration was reported in one study (Christmann 2002). None of the trials detected predefined levels of decreased urine output and increased levels of BUN and creatinine.
Authors' conclusions
The available data is insufficient to draw conclusions regarding the efficacy of continuous indomethacin infusion vs. bolus injections for the treatment of PDA. Although continuous indomethacin seems to cause less alterations in cerebral, renal and mesenteric circulations, the clinical meaning of this effect is unclear. Definitive recommendations about the preferred method of indomethacin administration in premature infants cannot be made based on the current findings of this review.
Plain language summary
Continuous infusion versus intermittent bolus doses of indomethacin for patent ductus arteriosus closure in symptomatic preterm infants
Patent ductus arteriosus (PDA) occurs when an artery near the heart and lungs stays open and does not close off after birth. Babies born early (preterm) have an increased risk of complications and death due to PDA. Indomethacin has been used to close the PDA; however, it can reduce blood flow in organs such as brain, kidneys and intestine. There is no agreement on the ideal dose and duration of treatment with indomethacin. In order to reduce the adverse effects of indomethacin on blood flow, some investigators have recommended administering the same total dose as a continuous infusion over 36 hours. In this review, the analysis of the two eligible trials found that the data was insufficient to reach a conclusion regarding the effectiveness of the 36‐hr continuous infusion method. The blood flow lowering side‐effects of indomethacin were reduced by the continuous infusion method, but there was insufficient data to recommend this administration method versus the traditional method.
Background
Description of the condition
Patent ductus arteriosus (PDA) is a common problem in the care of preterm infants. Failure of functional closure of the ductus leads to shunting of blood from the aorta to the pulmonary circulation as the pulmonary vascular resistance falls. The clinical consequences of a PDA are mainly related to the degree of left‐to‐right shunting through the ductus. Shunting results in alterations in blood flow distribution to the vital organs owing to a drop in diastolic pressure and localized vasoconstriction (Clyman 1996). Treatment may be necessary to prevent impaired organ perfusion that may result in serious complications such as necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH) and bronchopulmonary dysplasia (BPD) (Cotton 1978; Clyman 1996).
Description of the intervention
Indomethacin has been widely used for the prevention and treatment of hemodynamically significant PDAs (Friedman 1976; Clyman 1996). It has been shown that prophylactic indomethacin in very low birth weight infants reduces the incidence of symptomatic PDA, the need for surgical PDA ligation, and the incidence of IVH, including grades 3 and 4 (Fowlie 2002). Therapeutic indomethacin has been used in a variety of regimens to treat patients with symptomatic and presumed hemodynamically significant PDA (Nehgme 1992) with a reported efficacy of 66 to 80% (Gersony 1983; Lago 2002). Although indomethacin results in ductal closure in the majority of cases, it is ineffective in up to 40% of patients (Siassi 1976). Additionally, the ductus will reopen in 35% of those infants who initially respond to the drug (Gersony 1983). PDAs that fail to close with indomethacin may require surgical ligation.
There are potential adverse effects of indomethacin use in premature infants. Indomethacin induces a significant reduction in cerebral (van Bel 1989; Austin 1992), mesenteric (van Bel 1990) and renal (van Bel 1991; Pezzati 1999) blood flow velocities as measured by Doppler ultrasonography. These changes in organ blood flow, possibly caused by vasoconstriction, may result in impaired renal function and gastrointestinal problems such as spontaneous gastrointestinal perforation or NEC. Indomethacin can also cause decreased platelet function that might lead to coagulation disturbances. More recently, ibuprofen has been used with the same efficacy of indomethacin to close PDAs and was associated with fewer side effects (Shah 2003; van Overmeire 1997).
How the intervention might work
Factors known to influence the effectiveness of indomethacin therapy and the incidence of adverse effects are dose, timing of administration, birth weight and age of the patient being treated. However, the relationship of response to serum concentration and dose remains uncertain (Ment 1988; Shaffer 2002). Dosage and duration of indomethacin treatment have been addressed in several studies (Brook 1995). In one review, definitive recommendations about the preferred duration of therapy could not be reached based on a comparison of prolonged (four or more doses) versus short (three or less) courses of indomethacin (Herrera 2001). A short 3‐dose schedule has been widely used, but the optimal regimen of indomethacin has not yet been established (Christmann 2002). The effects of altering indomethacin infusion rates on its efficacy and side effects have been studied in a small number of studies; however, the results remain controversial. A 30‐minute infusion course compared to a rapid bolus (< 30 seconds) course to ascertain the effects of indomethacin on cerebral blood flow velocities revealed inconclusive results (Simko 1994): the two study groups showed no significant differences in requirement of further indomethacin therapy or surgical ligation to close the PDA. In another study, the decrease in cerebral blood flow velocity after indomethacin injection was eliminated by administering indomethacin as a continuous infusion over 36 hours (Hammerman 1995). This study also demonstrated that continuous (36 hours) and rapid (≤ 1 minute) administration of indomethacin appeared to be equally efficacious in mediating ductal closure.
Why it is important to do this review
At present, there is no consensus on optimal dosage and duration of indomethacin therapy for the treatment of PDA. Additionally, little is known regarding the influence on side effects caused by manipulating dosage and infusion rates.
Objectives
To compare the efficacy and safety of continuous infusion of indomethacin versus intermittent bolus infusions in closing the symptomatic PDA in preterm infants.
Secondary objectives included a review of complications that may be associated with these treatment regimens. In subgroup analyses, the effectiveness and safety of indomethacin to close a PDA was examined in relation to the following criteria:
Gestational age (< 28 weeks, 28 ‐ 32 weeks, 33 to 37 weeks)
Birth weight (< 1000 grams, 1000 to 1500 grams, 1501 to 2500 grams)
Dose (≤ 0.3 mg/kg total dose vs. > 0.3 mg/kg), and
Method used to diagnose a PDA (by ECHO criteria or only by clinical criteria)
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials
Types of participants
Preterm infants less than 37 weeks estimated gestation with a symptomatic PDA diagnosed clinically and/or by echocardiographic examination in the neonatal period (< 28 d). Patients were considered to have clinically symptomatic PDA when a combination of clinical signs and radiographic findings were evident. These signs and findings included the presence of a continuous murmur, hyperactive precordium, widened pulse pressure, tachycardia (heart rate > 170/min), tachypnea (respiratory rate > 70/min) and cardiomegaly with signs of pulmonary congestion on the chest radiograph. Infants were eligible if the PDA was diagnosed by echocardiogram with or without Doppler ultrasound, even when the clinical constellation was absent.
Types of interventions
Experimental group: Continuous infusion of indomethacin given after 24 hours of life for closure of a symptomatic PDA. All doses and durations of any continuous infusion were included. Control group: Indomethacin administered as a bolus dose of no longer than 20 minutes in any dosing schedule after 24 hours of life for closure of a symptomatic PDA.
Types of outcome measures
Primary outcomes
Failure of PDA to close after completion of allocated treatment (assessed at the end of treatment)
Secondary outcomes
Re‐opening of PDA after initial closure with allocated treatment
Re‐treatment with indomethacin
Requirement of surgical ligation
Neonatal mortality (death during the first 28 days of life)
Mortality (death prior to initial hospital discharge)
BPD (oxygen therapy at 36 weeks postmenstrual age)
Change in cerebral blood flow measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline)
IVH (all grades)
Severe IVH (grade III/IV)
ROP (defined by ICROP classification: any ROP and severe ROP stage 3 or worse)
Neurodevelopmental outcome (sensorineural hearing loss, visual impairment, cerebral palsy, developmental delay at 24 months corrected age assessed by a standardized and validated assessment tool and/or a child developmental specialist)
Change in renal blood flow measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline)
Change in urine output (oliguria defined as urine output defined as < 1 cc/kg/hr) during therapy
Change in BUN (> 20 mg/dl) levels
Change in creatinine (> 1.8 mg/dl) levels
Change in mesenteric blood flow measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline)
Isolated intestinal perforation (focal small bowel perforation, distinct from NEC, recognized by surgery)
NEC (Bell's stage 2 or greater)
Search methods for identification of studies
Electronic searches
A MEDLINE search was conducted from 1966 to March 2007, using Pub Med software using the following search terms as individual searches and in combination:
Exp indomethacin
infant, preterm, MeSH
Controlled clinical trial. pt
newborn: birth‐1 month
EMBASE was searched over the same period as for MEDLINE using a similar search strategy. The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2007) was searched with the text words "indomethacin" and "infant, preterm". The web site "clinical trials.gov" were searched for completed or ongoing trials related to therapeutic indomethacin for symptomatic PDA in premature infants. No language restrictions were applied.
In December 2009, we updated the search as follows: MEDLINE (search via PubMed), CINAHL, EMBASE and CENTRAL (The Cochrane Library) were searched from 2007 to 2009. Search term: indomethacin. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searching other resources
Bibliographies of reviews and trials were examined for references to other trials. Previous reviews including cross‐references, abstracts, and conference and symposia proceedings published in Pediatric Research (Pediatric Academic Societies Annual Meeting Abstract Book, 1972 to 2006) were reviewed.
Data collection and analysis
Data collection and analysis was done in accordance with the recommendations of the CNRG.
Selection of studies
The two review authors (ASG, RAE) obtained the full text of all studies of possible relevance for independent assessment. The review authors decided which studies met the inclusion criteria and graded the methodological quality.
Data extraction and management
One review author (ASG) entered data into RevMan. Authors were contacted to obtain the raw data for the analyses.
Assessment of risk of bias in included studies
The methodological quality of studies were documented using the recommended criteria of the Cochrane Neonatal Review Group: selection bias (how well allocation and randomization were blinded); performance bias (were the treating doctors masked to therapy); attrition bias (completeness of follow‐up); and detection bias (blind outcome assessment and blind assessment with the US scanning).
All four methodological criteria of the included trials were assessed using the Cochrane approach: Grade A: adequate concealment, Grade B: uncertain, Grade C: clearly inadequate concealment. This data was entered in the "Characteristics of Included Studies" table.
In addition, the following issues were evaluated and entered into the Risk of Bias Table:
1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
‐ adequate (any truly random process e.g. random number table; computer random number generator);
‐ inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number);
‐ unclear.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
‐ adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
‐ unclear.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
‐ adequate, inadequate or unclear for participants;
‐ adequate, inadequate or unclear for personnel;
‐ adequate, inadequate or unclear for outcome assessors.
In some situations there may be partial blinding e.g. where outcomes are self‐reported by unblinded participants but they are recorded by blinded personnel without knowledge of group assignment. Where needed “partial” was added to the list of options for assessing quality of blinding.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
‐ adequate (< 20% missing data);
‐ inadequate (≥ 20% missing data):
‐ unclear.
(5) Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
‐ adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
‐ inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
‐ unclear.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
‐ yes; no; or unclear.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Statistical analyses were performed using Review Manager software. Categorical data were analyzed using relative risk (RR), risk difference (RD) and the number needed to treat (NNT). Continuous data were analyzed using weighted mean difference (WMD). The 95% Confidence interval (CI) was reported on all estimates.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we planned to explore the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses.
Assessment of reporting biases
Funnel plots to identify publication bias were not examined due to insufficient number of trials.
Data synthesis
Meta‐analysis was performed using Review Manager software (RevMan 5) supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to evaluate the effectiveness and safety of indomethacin to close a PDA in relation to the following criteria:
Gestational age (< 28 weeks, 28 to 32 weeks, 33 to 37 weeks)
Birth weight (< 1000 grams, 1000 to 1500 grams, 1501 to 2500 grams)
Dose (≤ 0.3 mg/kg total dose vs. > 0.3 mg/kg), and
Method used to diagnose a PDA (by ECHO criteria or only by clinical criteria)
Subgroup analyses were not performed because data were unavailable.
Results
Description of studies
The search identified two studies that randomized infants less then 1750 g birth weight with PDA. Both studies compared the effects of a 36‐hours infusion of indomethacin versus a course of rapid bolus administrations (Christmann 2002, Hammerman 1995). Christmann 2002: In this two‐center trial 32 preterm infants (25 to 32 weeks gestational age) with echocardiographically documented PDA were randomized to receive the same total dose of indomethacin (0.4 mg/kg) either as three bolus injections or as a continuous infusion over 36 hours. In the bolus injection group, three intravenous injections were given rapidly within 30 seconds. The initial dose was 0.2 mg/kg followed by two doses of 0.1 mg/kg indomethacin at 12 and 36 hours after the first injection. Blood flow velocities of the internal carotid artery (ICA), renal artery (RA) and superior mesenteric artery (SMA) were measured before and at 10, 30, 60 and 120 minutes and 12, 24, 36 and 48 hours after initiation of indomethacin treatment. Echocardiographic assessments were done in a masked fashion by a cardiologist before and 24 hours after the completion of the treatment. Perinatal characteristics were registered. Primary outcome variables were PDA closure, reopening of PDA after completion of the allocated treatment, neonatal mortality, IVH, NEC and changes in blood flow velocities (ICA, RA and SMA) during indomethacin treatment. Other studied variables were BUN, creatinine, hemoglobin, hematocrit, platelet count, serum sodium, potassium, BUN, creatinine, blood gasses, ventilator settings, medications and exposure to phototherapy. Urine output and heart rate, arterial oxygen saturation, tc pO2, tc pCO2 and blood pressure were monitored continuously. Hammerman 1995: This two‐center trial enrolled 18 preterm infants < 1750 g (28 to 29 weeks gestational age) with echocardiographically documented PDA. Infants were randomized to receive indomethacin either by three rapid injections (an initial dose of 0.2 mg/kg followed by two doses of 0.1 mg/kg every 12 hours) or by continuous intravenous infusion (11 mcg/kg/hour) over 36 hours, providing an equivalent total dose. Blood flow velocity of the middle cerebral artery (MCA) was measured before and at four and 30 minutes and at 24 hours following completion of the initial infusion for the bolus group or the start of the continuous infusion for the continuous group by the investigator who was blinded to the group assignment. Echocardiograms were obtained daily for four days after entry to the study and then at one week of age. Perinatal characteristics were registered. Primary outcome variables were PDA closure, reopening of PDA after completion of the allocated treatment, IVH and changes in cerebral blood flow velocities during the indomethacin treatment. Other studied variables were systolic blood pressure, BUN and creatinine.
Risk of bias in included studies
Christmann 2002: Enrolled patients were randomized using the sealed envelope technique. Allocation concealment was unclear. Investigators who preformed echocardiograms and blood flow measurements were not reported to be blinded to group assignments. Apparently, there was a loss of three infants from both continuous and bolus groups. No explanation for these losses was given by the authors.
Hammerman 1995: This was a double‐blind study where the groups were assigned by computer. Allocation concealment was unclear. However, investigators performing echocardiograms and blood flow measurements were blinded to group assignments.
The assessment details of the studies are presented in the table "Characteristics of Included Studies".
Effects of interventions
CONTINUOUS vs. BOLUS INDOMETHACIN Two studies met the inclusion criteria.
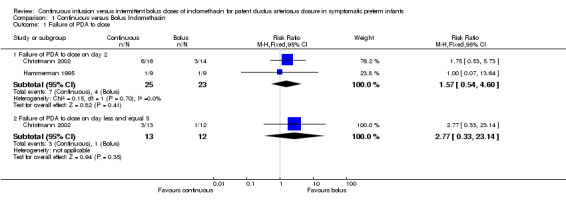
Primary Outcome: Failure of PDA to close after completion of allocated treatment assessed at the end of treatment (Outcome 1.1): This outcome was reported by both trials (Christmann 2002; Hammerman 1995). Both studies found no difference for PDA closure on day two. In the meta analysis, there was no significant difference between the continuous and bolus groups on day two. The typical estimates were RR 1.57 (95% CI 0.54, 4.60), RD 0.10 (95% CI ‐0.13, 0.33). Closure rates of PDA between groups for surviving infants less than five days of age, which was only reported in the study by Christmann 2002, was also nonsignificant [RR 2.77 (95% CI 0.33, 23.14), RD 0.15 (95% CI ‐0.13, 0.42)].
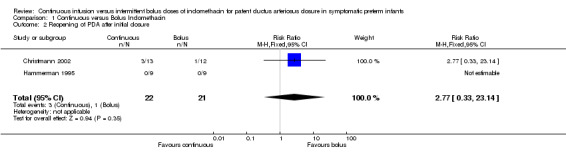
Secondary Outcomes: Reopening of PDA after initial closure with allocated treatment (Outcome 1.2): Both studies reported this outcome (Christmann 2002, Hammerman 1995). There was no statistical significance between the continuous and bolus groups in reopening of the PDA after the allocated treatment: [typical RR 2.77 (95% CI 0.33, 23.14), typical RD 0.09 (95% CI ‐0.10, 0.27)].
Retreatment with indomethacin: None of the studies reported this outcome.
Requirement of surgical ligation: None of the studies reported this outcome.
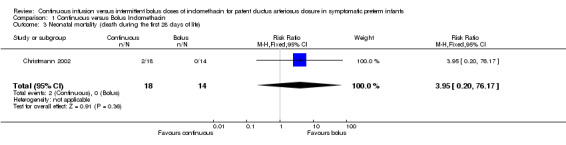
Neonatal mortality (death during the first 28 days of life) (Outcome 1.3): Only one study reported this outcome (Christmann 2002). There was no statistical difference between the groups for neonatal mortality [RR 3.95 (95% CI 0.20, 76.17), RD 0.11 (95% CI ‐0.07, 0.29)].
Mortality (death prior to initial hospital discharge): None of the studies reported this outcome BPD (oxygen therapy at 36 weeks postmenstrual age): None of the studies reported this outcome.
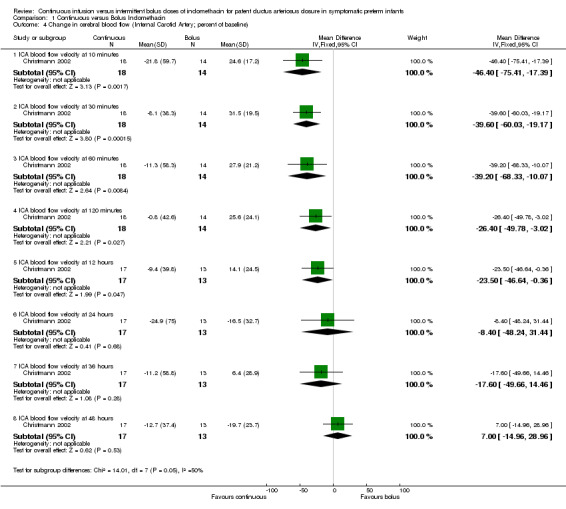
Change in cerebral blood flow velocity measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline) (Outcomes 1.4 and 1.5): Two studies reported this outcome but on different arteries for which these analyses were carried out separately: One study examined the ICA (Christmann 2002) and the other used the MCA (Hammerman 1995). Both studies showed a decrease in the cerebral mean blood low velocities after the bolus injections until 24 hours post‐administration compared to no decrease in the continuous groups.
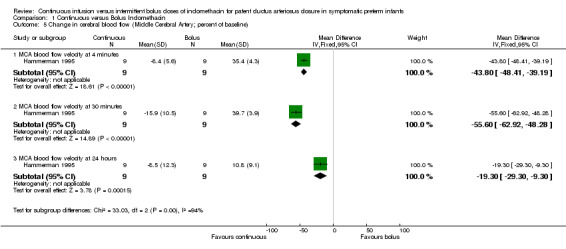
The study by Christmann (Christmann 2002) showed that the mean flow velocities remained similar to baseline measurements in the continuous group; however, they decreased after the bolus injection. A significant difference between the bolus and the continuous groups during the first 12 hours was noted (Table 01.04) . This difference gradually lessened and became statistically insignificant at 24 hours [MD ‐46.40 (95% CI ‐75.41, ‐17.39) at 10 minutes; MD ‐39.60 (95% CI ‐60.03, ‐19.17) at 30 minutes; MD ‐39.20 (95% CI ‐68.33, ‐10.07) at 60 minutes; MD ‐26.40 (95% CI ‐49.78, ‐3.02) at 120 minutes; MD ‐23.50 (95% CI ‐46.64, ‐0.36) at 12 hours; MD ‐8.40 (95% CI ‐48.24, 31.44) at 24 hours; MD ‐17.60 (95% CI ‐49.66, 14.46) at 36 hours; MD 7.00 (95% CI ‐14.96, 28.96) at 48 hours]. In the second study (Hammerman 1995), the mean flow velocities remained similar to baseline measurements in the continuous group; however, they decreased after the bolus injection (Table 01.05). The difference continued to be significant during the entire 24 hours of the study [MD ‐43.80 (95% CI ‐48.41, ‐39.19) at 4 minutes; MD ‐55.60 (95% CI ‐62.92, ‐48.28) at 30 minutes; MD ‐19.30 (95% CI ‐29.30, ‐9.30) at 24 hours]. IVH (all grades) (Outcome 1.6): Both studies reported this outcome but none of the infants were found to have IVH.
Severe IVH (grade III/IV): None of the studies reported this outcome.
ROP (defined by ICROP classification: any ROP and severe ROP stage 3 or worse): None of the studies reported this outcome.
Neurodevelopmental outcome (sensorineural hearing loss, visual impairment, cerebral palsy, developmental delay at 24 months corrected age assessed by a standardized and validated assessment tool and/or a child developmental specialist): None of the studies reported this outcome.
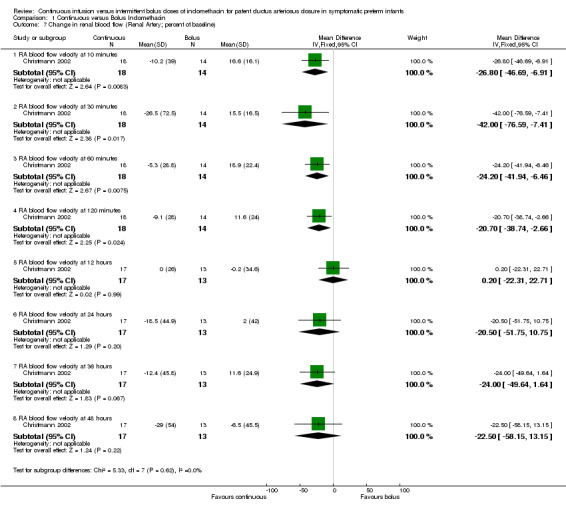
Change in renal blood flow measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline) (Outcome 1.7): Only one study reported on renal blood flow (Christmann 2002). In the bolus group, blood flow in the renal circulation decreased significantly within 10 minutes after indomethacin injection and remained significantly lower than the bolus group until 12 hours. The estimates were MD ‐26.80 (95% CI ‐46.69, ‐6.91) at 10 minutes; MD ‐42.00 (95% CI ‐76.59, ‐7.41) at 30 minutes; MD ‐24.20 (95% CI ‐41.94, ‐6.46) at 60 minutes; MD ‐20.70 (95% CI ‐38.74, ‐2.66) at 120 minutes; MD 0.20 (95% CI ‐22.31, 22.71) at 12 hours; MD ‐20.50 (95% CI ‐51.75, 10.75) 24 hours; MD ‐24.00 (95% CI ‐49.64, 1.64) at 36 hours; MD ‐22.50 (95% CI ‐58.15, 13.15) at 48 hours.
Change in urine output (oliguria defined as urine output defined as < 1 cc/kg/hr or compared to baseline) during therapy (Outcome 1.8): Only one of the two studies reported on urine output (Christmann 2002). This study documented no oliguria, which was defined as urine output less than 1 cc/kg/hr. Change in BUN (> 20 mg/dl or compared to baseline) (Outcome 1.9): Two studies reported on BUN (Christmann 2002; Hammerman 1995). There was no detected value of BUN > 20 mg/dl.
Change in creatinine (> 1.8 mg/dl or compared to baseline) (Outcome 1.10): Two studies reported this outcome (Christmann 2002; Hammerman 1995). There were no infants observed with this outcome beyond the cutoff level of 1.8 mg/dl.
Change in mesenteric blood flow measured by Doppler ultrasound during allocated treatment period (either absolute or as a percentage of baseline) (Outcome 1.11): Only one of the two studies reported on mesenteric blood flow (Christmann 2002). In the bolus group, blood flow in the mesenteric circulation decreased significantly within 10 minutes after indomethacin injection and remained significantly low until 24 hours. Blood flow then increased to a level close to the continuous group and the significance disappeared. The estimates were MD ‐32.00 (95% CI ‐53.01, ‐10.99) at 10 minutes; MD ‐26.50 (95% CI ‐45.34, ‐7.66) at 30 minutes; MD ‐21.70 (95% CI ‐43.29, ‐0.11) at 60 minutes, MD ‐24.80 (95% CI ‐47.06, ‐2.54) at 120 minutes; MD ‐2.90 (95% CI ‐52.56, 46.76) at 12 hours; MD ‐7.40 (95% CI ‐71.40, 56.60) 24 hours; MD ‐11.10 (95% CI ‐44.58, 22.38) at 36 hours; MD ‐26.70 (95% CI ‐94.78, 41.38) at 48 hours. Isolated intestinal perforation (focal small bowel perforation, distinct from NEC, recognized by surgery): None of the studies reported this outcome.
NEC (Bell's stage 2 or greater) (Outcome 1.12): Only one study reported this outcome (Christmann 2002). There was no statistical significance in NEC between the groups [RR .0.56 (95% CI 0.03, 12.33), RD ‐0.07 (95% CI ‐0.28, 0.14)]. Subgroups analyses: Pre‐planned subgroups analyses were not possible because data were not available separately regarding gestational age and birth weight. The dose of indomethacin used was the same in both studies and each study used echocardiography to diagnose PDA.
Discussion
An extensive literature search identified only two small trials eligible for this review. Considering the small number of events in these two trials, the data analysis was found to be insufficient to draw conclusions from most outcomes investigated. The analysis of the main outcome of "failed PDA closure" showed that the comparison slightly favored bolus versus continuous indomethacin on both day two and five. The findings were not statistically significant. Similar trends were noted for the secondary outcomes of reopening of PDA and neonatal mortality but did not reach statistical significance. The analysis of the outcome for NEC favored continuous indomethacin; however, the analysis was statistically insignificant. Comparison for IVH could not be estimated due to no reported cases of IVH in each group. For all the outcomes, the number of observed events were so few that no reliable statement about relative effectiveness of continuous versus bolus indomethacin administration could be made. Neither study reported on requirements for retreatment with indomethacin, surgical ligation of PDA, BPD, ROP, isolated intestinal perforation or neurodevelopmental outcome.
The current review demonstrates that bolus administration of indomethacin compared to continuous infusion caused a significant reduction in cerebral blood flow. The maximum decrease in cerebral blood flow velocity following the bolus administration took place within the first 30 minutes and was approximately 35 to 40% of the baseline values. The flow decrease started to improve after 30 minutes; however, the difference remained statistically significant until 12 to 24 hours post‐administration. Although each study examined a different artery, namely ICA (Christmann 2002) and MCA (Hammerman 1995), the results were similar. In premature infants, it has been shown that indomethacin can cause reductions in cerebral artery blood flow ranging from 25 to 60% as measured by Doppler (Mardoum 1991; Laudignon 1988; Evans 1987; van Bel 1989). Although it is not known at what level flow decrease should be considered dangerous, any significant drop in the cerebral blood flow is disturbing because it may be a contributing cause of brain underperfusion or ischemia in premature infants. It is hypothesized that a decrease in cerebral blood flow could potentially increase the incidence of periventricular leukomalacia. It has also been shown that this decrease of blood flow may be advantageous because it might lower the incidence and severity of IVH when indomethacin is used prophylactically (Ment 1993). However, based on the current studies in the literature, it is impossible to determine the pathogenesis of ischemic brain damage and the clinical significance of indomethacin‐mediated blood flow decrease in preterm infants.
In this review, only one trial with a small number of patients reported on the effects of indomethacin on mesenteric and renal blood flows (Christmann 2002). Initially, there was a significant decrease in the blood flow to the mesenteric artery and the renal artery in the bolus group. This effect gradually disappeared by 12 hours. Changes in urine output were reported by one trial (Christmann 2002); however, there was no documented oliguria. Changes in BUN and creatinine levels were reported by both trials (Christmann 2002; Hammerman 1995). However, there was no value above the predefined abnormal levels for both BUN and creatinine. Based on these results originating mostly from a single study, it would be difficult to reach a generalizable conclusion regarding the clinical consequences of reductions in mesenteric and renal blood flow. The well‐known adverse effect of a decrease in blood flow to the mesenteric and renal circulations following indomethacin injection has always been a concern. This flow reduction may potentially be associated with deterioration in renal function, as evidenced by decreased urine output and increased BUN and creatinine as well as isolated intestinal perforation and NEC. However, none of these adverse effects were found in either of the studies included in this review and evidence does not favor either therapy in this regard.
There are several studies that have compared the effects of different indomethacin administration regimens on decreasing blood flow in preterm infants. Simko et al studied bolus and 30 minute infusion rates. Interestingly, both 30‐minute and bolus infusion groups had significantly decreased cerebral flow velocities, although the bolus infusion group dropped more rapidly and fell to lower levels (Simko 1994). Edwards et al compared the effects of rapid (30 seconds) versus slow (20 to 30 minutes) infusions of indomethacin on cerebral flow by near‐infrared spectroscopy (Edwards 1990). They found an equal cerebral blood flow decrease in both groups. Colditz et al compared the effects of a slow infusion of 20 minutes with a five minute rapid infusion of indomethacin (Colditz 1989). They showed a significant reduction in the cerebral blood flow velocity with the rapid infusion, which was absent in the slow infusion group. Lastly, Austin et al studied the effects of a 30‐minute indomethacin infusion on cerebral hemodynamics (Austin 1992). They had no rapid infusion group as control, but noted that the slow infusion was associated with a significant decrease in the cerebral flow (Austin 1992). In summary, it appears that slowing the infusion to a period of 20 to 30 minutes produces some improvement in the indomethacin‐associated reduction of the cerebral blood flow; however, it does not completely eliminate it. Slowing the infusion rate further down to 36‐hours, such as discussed in the studies of this review, might be an option to prevent such cerebral hemodynamic fluctuations. However, the clinical consequences of these flow fluctuations are unknown. The use of continuous infusion of indomethacin might also present some disadvantages in care of the preterm infant such as the need for an intravenous site for 36 hours and the requirement of a larger amount of fluid in order to provide lower concentration of indomethacin. The aim of treatment with indomethacin is to close a PDA. The two small trials included in this review did not yield sufficient evidence to recommend a protocol change in the treatment of PDA favoring the use of continuous infusion of indomethacin over bolus infusion. A recent retrospective case‐control study by de Vries et al reported that continuous infusion might be less effective in closing a PDA than bolus infusions due to low plasma levels for ductal closure, especially in infants < 1000 g and/or precipitation of indomethacin in solutions prepared with glucose (de Vries 2005). It is apparent that further studies are needed to investigate the efficacy of a continuous indomethacin regime as well as clinical outcomes associated with blood flow reduction in cerebral, mesenteric and renal circulations. These studies should also include long‐term neurodevelopmental follow‐up of at least 18 months corrected age in order to clarify the consequences of the adverse effects related to indomethacin.
Authors' conclusions
Implications for practice.
Considering the paucity of events in these two eligible trials, the available data was found to be insufficient to draw conclusions regarding the efficacy of continuous indomethacin versus bolus administration for the treatment of PDA. The adverse affect of indomethacin on blood flow in cerebral, mesenteric and renal circulations seems to be reduced by continuous infusion. However, there is a lack of data on the clinical significance of this adverse effect and its amelioration by any method of administration. Considering the small sample size of these two studies, data seems insufficient to reach a conclusion about the preferred methods of indomethacin administration in favor of continuous versus bolus infusions.
Implications for research.
There is a lack of controlled trials on administration methods and potential complications of indomethacin use. The reviews comparing prolonged vs. short courses and continuous versus bolus infusions have failed to yield conclusions due to the small number of subjects recruited in these trials. Additional trials will be needed to establish comparable therapeutic efficacy of continuous infusion of indomethacin treatment. Clinical implications of indomethacin‐associated reduction of cerebral, mesenteric and renal blood flow in preterm infants should be further investigated. These trials should investigate not only short‐term outcomes such as reduction of cerebral, mesenteric and renal blood flow and mortality but also long‐term outcomes such as ROP, BPD and neurodevelopmental outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 3 February 2010 | New search has been performed | This review updates the existing review "Continuous infusion versus intermittent bolus doses of indomethacin for patent ductus arteriosus closure in symptomatic preterm infants" published in the Cochrane Database of Systematic Reviews (Görk 2008). Updated search found no new trials. No changes to conclusions. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 15 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Continuous versus Bolus Indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of PDA to close | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Failure of PDA to close on day 2 | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.54, 4.60] |
| 1.2 Failure of PDA to close on day less and equal 5 | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.33, 23.14] |
| 2 Reopening of PDA after initial closure | 2 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.33, 23.14] |
| 3 Neonatal mortality (death during the first 28 days of life) | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.20, 76.17] |
| 4 Change in cerebral blood flow (Internal Carotid Artery; percent of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 ICA blood flow velocity at 10 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐46.40 [‐75.41, ‐17.39] |
| 4.2 ICA blood flow velocity at 30 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐39.6 [‐60.03, ‐19.17] |
| 4.3 ICA blood flow velocity at 60 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐39.2 [‐68.33, ‐10.07] |
| 4.4 ICA blood flow velocity at 120 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐26.40 [‐49.78, ‐3.02] |
| 4.5 ICA blood flow velocity at 12 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐23.50 [‐46.64, ‐0.36] |
| 4.6 ICA blood flow velocity at 24 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐8.40 [‐48.24, 31.44] |
| 4.7 ICA blood flow velocity at 36 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐17.6 [‐49.66, 14.46] |
| 4.8 ICA blood flow velocity at 48 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐14.96, 28.96] |
| 5 Change in cerebral blood flow (Middle Cerebral Artery; percent of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 MCA blood flow velocity at 4 minutes | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐43.8 [‐48.41, ‐39.19] |
| 5.2 MCA blood flow velocity at 30 minutes | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐55.6 [‐62.92, ‐48.28] |
| 5.3 MCA blood flow velocity at 24 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐19.3 [‐29.30, ‐9.30] |
| 6 IVH (all grades) | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in renal blood flow (Renal Artery; percent of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 RA blood flow velocity at 10 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐26.8 [‐46.69, ‐6.91] |
| 7.2 RA blood flow velocity at 30 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐42.00 [‐76.59, ‐7.41] |
| 7.3 RA blood flow velocity at 60 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐24.2 [‐41.94, ‐6.46] |
| 7.4 RA blood flow velocity at 120 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐20.7 [‐38.74, ‐2.66] |
| 7.5 RA blood flow velocity at 12 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.2 [‐22.31, 22.71] |
| 7.6 RA blood flow velocity at 24 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐20.5 [‐51.75, 10.75] |
| 7.7 RA blood flow velocity at 36 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐24.0 [‐49.64, 1.64] |
| 7.8 RA blood flow velocity at 48 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐22.50 [‐58.15, 13.15] |
| 8 Changes in urine output (oliguria defined as urine output <1 cc/kg/hr) during therapy | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in BUN (>20 mg/dl) levels | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Change in creatinine (>1.8 mg/dl) levels | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Change in mesenteric blood flow (Superior Mesenteric Artery, percent of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 SMA blood flow velocity at 10 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐32.0 [‐53.01, ‐10.99] |
| 11.2 SMA blood flow velocity at 30 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐26.50 [‐45.34, ‐7.66] |
| 11.3 SMA blood flow velocity at 60 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐21.70 [‐43.29, ‐0.11] |
| 11.4 SMA blood flow velocity at 120 minutes | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐24.8 [‐47.06, ‐2.54] |
| 11.5 SMA blood flow velocity at 12 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐52.56, 46.76] |
| 11.6 SMA blood flow velocity at 24 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐7.40 [‐71.40, 56.60] |
| 11.7 SMA blood flow velocity at 36 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐11.10 [‐44.58, 22.38] |
| 11.8 SMA blood flow velocity at 48 hours | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐26.70 [‐94.78, 41.38] |
| 12 NEC (Bell's stage 2 or greater) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.03, 12.23] |
1.1. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 1 Failure of PDA to close.
1.2. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 2 Reopening of PDA after initial closure.
1.3. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 3 Neonatal mortality (death during the first 28 days of life).
1.4. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 4 Change in cerebral blood flow (Internal Carotid Artery; percent of baseline).
1.5. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 5 Change in cerebral blood flow (Middle Cerebral Artery; percent of baseline).
1.6. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 6 IVH (all grades).
1.7. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 7 Change in renal blood flow (Renal Artery; percent of baseline).
1.8. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 8 Changes in urine output (oliguria defined as urine output <1 cc/kg/hr) during therapy.
1.9. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 9 Change in BUN (>20 mg/dl) levels.
1.10. Analysis.
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 10 Change in creatinine (>1.8 mg/dl) levels.
1.11. Analysis.
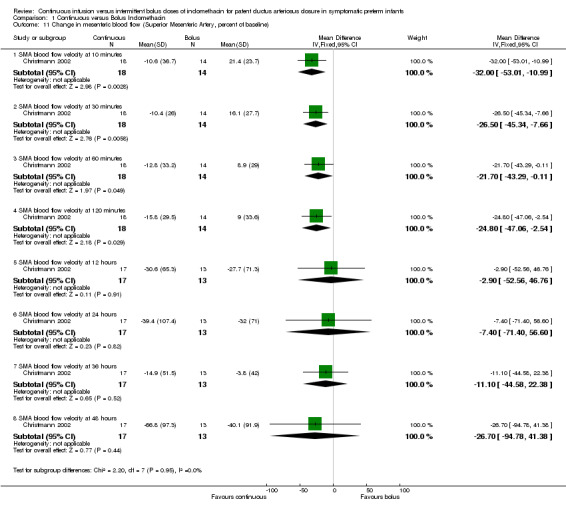
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 11 Change in mesenteric blood flow (Superior Mesenteric Artery, percent of baseline).
1.12. Analysis.
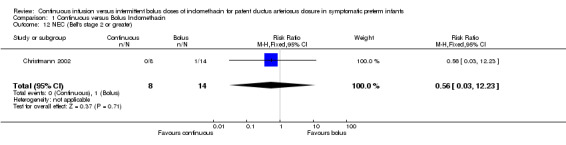
Comparison 1 Continuous versus Bolus Indomethacin, Outcome 12 NEC (Bell's stage 2 or greater).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Christmann 2002.
| Methods | Two‐center, randomized, controlled trial I. Blinding of randomization ‐ can't tell II. Blinding of intervention ‐ no III. Complete follow‐up ‐ no IV. Blinding of outcome measurements ‐ can't tell | |
| Participants | Study conducted in The Netherlands 32 infants (26‐35 weeks) with echocardiographic evidence of PDA were randomized. 18 infants, mean GA 29.4±0.5 weeks, >33 weeks 1, BW 1150±77 g, postnatal age 4±0.7 d (range 1‐14 d) were treated with continuous indomethacin. 14 infants, mean GA 30.8±0.5 weeks, >33 weeks 2, BW 1424±150 g, postnatal age 5±1.4 d (range 2‐22 d) were treated with bolus indomethacin. | |
| Interventions | Both groups received the same total amount of indomethacin (0.4mg/kg). In the continuous group, indomethacin was administered during 36 h period at a dose of 0.011 mg/kg/h, intravenously. In the bolus group, indomethacin was administered intravenously in three doses, rapidly within 30 seconds: Initial injection dose was 0.2 mg/kg and the subsequent doses were 0.1 mg/kg at 12 and 36 h after the initial dose. | |
| Outcomes | PDA closure Reopening of PDA after completion of the allocated treatment Neonatal mortality IVH NEC Blood flow velocities: ICA, RA and SMA ‐ before indomethacin and at 10, 30, 60, 120 minutes and 12, 24, 36 and 48 hours after initiation of indomethacin therapy Hemoglobin Hematocrit Platelet count Serum sodium, potassium, BUN and creatinine Blood gasses Ventilator settings Medications Exposure to phototherapy Urine output HR BP Arterial oxygen saturation tcpO2, tcpCO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Blinding of randomization ‐ can't tell |
| Blinding? All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome measurements ‐ can't tell |
| Incomplete outcome data addressed? All outcomes | High risk | Complete follow‐up ‐ no |
Hammerman 1995.
| Methods | Two‐center, randomized, controlled trial I. Blinding of randomization ‐ can't tell II. Blinding of intervention ‐ no III. Complete follow‐up ‐ no IV. Blinding of outcome measurements ‐ yes | |
| Participants | Study conducted in Israel 18 premature infants (BW 700‐1750 g) with echocardiographic evidence of PDA were randomized. 9 infants, mean GA 29±2 weeks, BW 1200±300 g were treated with continuous indomethacin. 9 infants, mean GA 28±2 weeks, BW 1100±200 g, were treated with bolus indomethacin. | |
| Interventions | In the continuous group, indomethacin was administered during 36 h period at a dose of 0.011 mg/kg/h, intravenously. In the bolus group, indomethacin was administered intravenously in three doses, rapidly within 1 minute: Initial injection dose was 0.2 mg/kg and the subsequent doses were 0.1 mg/kg every 12 hours. | |
| Outcomes | PDA closure by echocardiogram: at 4 day after the study and at 1 week of age Reopening of PDA after completion of the allocated treatment IVH MCA blood flow velocity: 4 and 30 minutes and 24 hours after initiation of indomethacin Systolic BP BUN Creatinine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Blinding of randomization ‐ can't tell |
| Blinding? All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome measurements ‐ yes |
| Incomplete outcome data addressed? All outcomes | High risk | Complete follow‐up ‐ no |
Contributions of authors
ASG ‐ Wrote the protocol, searched for the studies, extracted and analysed the data, contacted authors for additional data, wrote the review RAE ‐ Edited the protocol, searched for the studies, analysed the data, edited the review, provided clinical perspective and general advice MBB ‐ Edited the protocol, provided methodological and statistical advice, edited the review
The December 2009 was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll). This update was reviewed and approved by ASG.
Sources of support
Internal sources
Department of Pediatrics, Yale University, New Haven, USA.
External sources
No sources of support supplied, USA.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Christmann 2002 {published data only}
- Christmann V, Liem KD, Semmekrot BA, Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatrica 2002;91:440‐6. [DOI] [PubMed] [Google Scholar]
Hammerman 1995 {published data only}
- Hammerman C, Glaser J, Schimmel MS, Ferber B, Kaplan M, Eidelman AI. Continuous versus multiple rapid infusions of indomethacin: effects on cerebral blood flow velocity. Pediatrics 1995;95:244‐8. [PubMed] [Google Scholar]
Additional references
Austin 1992
- Austin NC, Pairaudeau PW, Hames TK, Hall MA. Regional cerebral blood flow velocity changes after indomethacin infusion in preterm infants. Archives of Disease in Childhood 1992;67:851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 1995
- Brook M, Heymann M. Patent ductus arteriosus. Heart Disease in Infants, Children and Adolescents including the Fetus and Young Adult. Williams and Wilkins, 1995. [Google Scholar]
Clyman 1996
- Clyman RI. Recommendation for the postnatal use of indomethacin: an analysis of four separate treatment strategies. Journal of Pediatrics 1996;128:601‐7. [DOI] [PubMed] [Google Scholar]
Colditz 1989
- Colditz P, Murphy D, Rolfe P, Wilkinson AR. Effect of infusion rate of indomethacin on cerebrovascular responses in preterm neonates. Archives of Disease in Childhood 1989;64:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 1978
- Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. Journal of Pediatrics 1978;93:647‐51. [DOI] [PubMed] [Google Scholar]
de Vries 2005
- Vries KS, Jagroep FK, Jaarsma AS, Elzenga NJ, Bos AF. Continuous indomethacin infusion may be less effective than bolus infusion for ductal closure in very low birth weight infants. American Journal of Perinatology 2005;22:71‐5. [DOI] [PubMed] [Google Scholar]
Edwards 1990
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, Reynolds EO. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 1990;335:1491‐5. [DOI] [PubMed] [Google Scholar]
Evans 1987
- Evans DH, Levene MI, Archer LN. The effect of indomethacin on cerebral blood‐flow velocity in premature infants. Developmental Medicine and Child Neurology 1987;29:776‐82. [DOI] [PubMed] [Google Scholar]
Fowlie 2002
- 10.1002/14651858.CD000174Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000174] [DOI] [PubMed] [Google Scholar]
Friedman 1976
- Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. New England Journal of Mediciine 1976;295:526‐9. [DOI] [PubMed] [Google Scholar]
Gersony 1983
- Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. Journal of Pediatrics 1983;102:895‐906. [DOI] [PubMed] [Google Scholar]
Herrera 2001
- Herrera C, Holberton J, Davis P. Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003480.pub2] [DOI] [Google Scholar]
Lago 2002
- Lago P, Bettiol T, Salvadori S, Pitassi I, Vianello A, Chiandetti L, Saia OS. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. European Journal of Pediatrics 2002;161:202‐7. [DOI] [PubMed] [Google Scholar]
Laudignon 1988
- Laudignon N, Chemtob S, Bard H, Aranda JV. Effect of indomethacin on cerebral blood flow velocity of premature newborns. Biology of the Neonate 1988;54:254‐62. [DOI] [PubMed] [Google Scholar]
Mardoum 1991
- Mardoum R, Bejar R, Merritt AT, Berry C. Controlled study of the effects of indomethacin on cerebral blood flow velocities in newborn infants. Journal of Pediatrics 1991;118:112‐5. [DOI] [PubMed] [Google Scholar]
Ment 1988
- Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor KJ, Scott DT, Gettner P, Sherwonit E, Williams J. Randomized low‐dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. Journal of Pediatrics 1988;112:948‐55. [DOI] [PubMed] [Google Scholar]
Ment 1993
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Schneider K, Katz KH, Taylor KJ, Duncan CC, Makuch RW. Risk period for intraventricular hemorrhage of the preterm neonate is independent of gestational age. Seminars in Perinatology 1993;17:338‐41. [PubMed] [Google Scholar]
Nehgme 1992
- Nehgme RA, O'Connor TZ, Lister G, Bracken MB. Patent ductus arteriosus. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:281‐324. [Google Scholar]
Pezzati 1999
- Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1999;135:733‐8. [DOI] [PubMed] [Google Scholar]
Shaffer 2002
- Shaffer CL, Gal P, Ransom JL, Carlos RQ, Smith MS, Davey AM, Dimaguila MA, Brown YL, Schall SA. Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Critical Care Medicine 2002;30:343‐8. [DOI] [PubMed] [Google Scholar]
Shah 2003
- Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004213] [DOI] [PubMed] [Google Scholar]
Siassi 1976
- Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low‐birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics 1976;57:347‐51. [PubMed] [Google Scholar]
Simko 1994
- Simko A, Mardoum R, Merritt TA, Bejar R. Effects on cerebral blood flow velocities of slow and rapid infusion of indomethacin. Journal of Perinatology 1994;14:29‐35. [PubMed] [Google Scholar]
van Bel 1989
- Bel F, Bor M, Stijnen T, Baan J, Ruys JH. Cerebral blood flow velocity changes in preterm infants after a single dose of indomethacin: duration of its effect. Pediatrics 1989;84:802‐7. [PubMed] [Google Scholar]
van Bel 1990
- Bel F, Zoeren D, Schipper J, Guit GL, Baan J. Effect of indomethacin on superior mesenteric artery blood flow velocity in preterm infants. Journal of Pediatrics 1990;116:965‐70. [DOI] [PubMed] [Google Scholar]
van Bel 1991
- Bel F, Guit GL, Schipper J, Bor M, Baan J. Indomethacin‐induced changes in renal blood flow velocity waveform in premature infants investigated with color Doppler imaging. Journal of Pediatrics 1991;118:621‐6. [DOI] [PubMed] [Google Scholar]
van Overmeire 1997
- Overmeire B, Follens I, Hartman S, Creten WL, Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Archives of Disease in Childhood Neonatal Edition 1997;76:F179‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Görk 2008
- Görk AS, Ehrenkranz RA, Bracken MB. Continuous infusion versus intermittent bolus doses of indomethacin for patent ductus arteriosus closure in symptomatic preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006071.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


