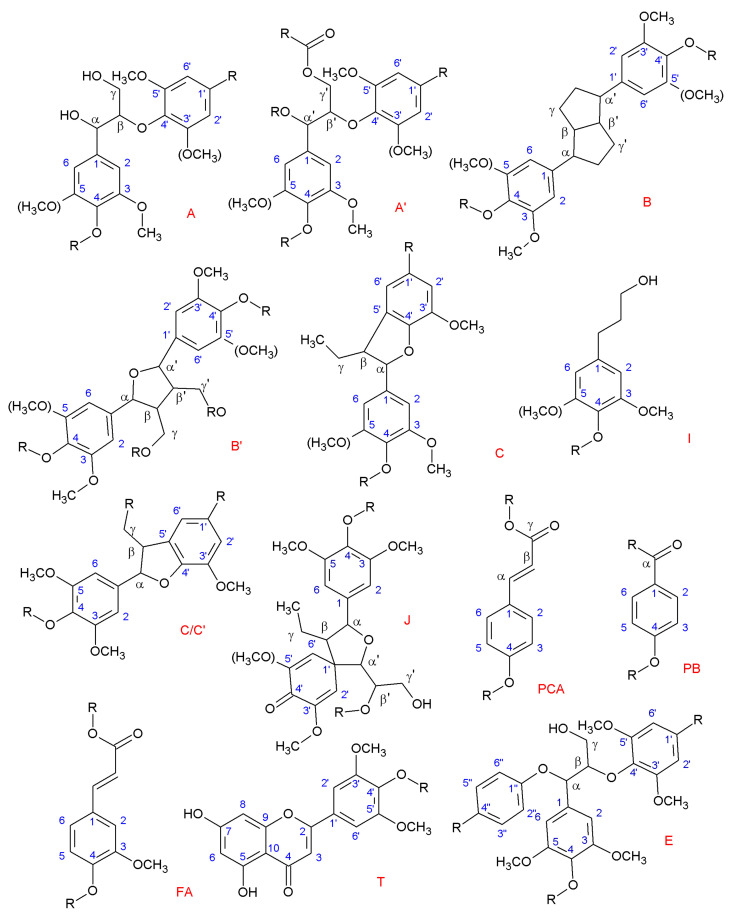
Figure 3.
Main lignin structures identified by NMR. (R may indicate both aliphatic and aromatic chains.) (A) β-O-4 alkyl-aryl ethers; (A’) β-O-4 alkyl-aryl ethers with acylated γ’-OH with p-coumaric acid; (B) resinols; (B’) di-c-acylated mono-tetrahydrofuran structure formed by β–β’ coupling and subsequent a-O-a’ bonding (R, acetyl/p-coumaroyl); (C) phenylcoumarans; (I) p-hydroxycinnamyl alcohol end-groups; (C/C’) γ-acetylated phenylcoumaran (R, acetyl) (J) spirodienones (β-1′); (PCA) p-coumarates; (PB) p-hydroxybenzoate; (FA) ferulates; (T) tricin incorporation into the lignin polymer through a G-type β-O-4 linkage; (E) α,β-diaryl ethers (α-O-4/β-O-4).