Abstract
Horizontal gene transfer (HGT) can allow traits that have evolved in one bacterial species to transfer to another. This has potential to rapidly promote new adaptive trajectories such as zoonotic transfer or antimicrobial resistance. However, for this to occur requires gaps to align in barriers to recombination within a given time frame. Chief among these barriers is the physical separation of species with distinct ecologies in separate niches. Within the genus Campylobacter, there are species with divergent ecologies, from rarely isolated single-host specialists to multihost generalist species that are among the most common global causes of human bacterial gastroenteritis. Here, by characterizing these contrasting ecologies, we can quantify HGT among sympatric and allopatric species in natural populations. Analyzing recipient and donor population ancestry among genomes from 30 Campylobacter species, we show that cohabitation in the same host can lead to a six-fold increase in HGT between species. This accounts for up to 30% of all SNPs within a given species and identifies highly recombinogenic genes with functions including host adaptation and antimicrobial resistance. As described in some animal and plant species, ecological factors are a major evolutionary force for speciation in bacteria and changes to the host landscape can promote partial convergence of distinct species through HGT.
Research organism: Other
Introduction
It is well established that bacteria do not conform to a strict clonal model of reproduction but engage in regular horizontal gene transfer (HGT) (Smith et al., 1991). This lateral exchange of DNA can confer new functionality on recipient genomes, potentially promoting novel adaptive trajectories such as colonization of a new host or the emergence of pathogenicity (Sheppard et al., 2018). In some cases, gene flow can occur at such magnitude, even between different species (Shapiro et al., 2016; Doolittle and Zhaxybayeva, 2009), that one may question why disparate lineages do not merge and why distinct bacterial species exist at all (Doolittle and Papke, 2006). An answer to this lies in considering the successive processes that enable genes from one strain to establish in an entirely new genetic background.
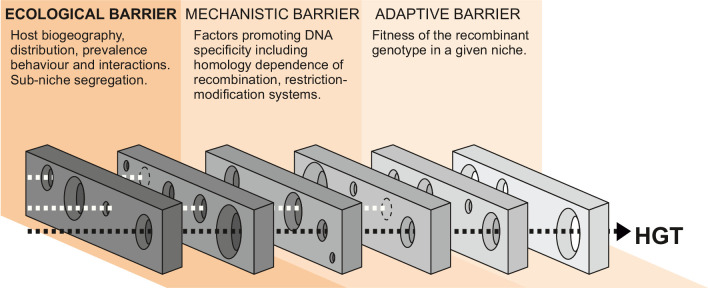
The probability of HGT is governed by the interaction of multiple factors, including exposure to DNA, the susceptibility of the recipient genome to DNA uptake, and the impact of recombined DNA on the recipient strain. These factors can be broadly defined in three functional phases, and HGT can only occur when gaps align in each successive ecological, mechanistic, and adaptive barrier within a given time frame (Figure 1). In the first phase, the quantity of DNA available to recipient strains is determined by ecological factors such as the distribution, prevalence, and interactions of donor and recipient bacteria, as well as the capacity for free DNA to be disseminated among species/strains. In the second phase, there are mechanistic barriers to HGT imposed by the homology dependence of recombination (Fraser et al., 2007) or other factors promoting DNA specificity – such as restriction-modification, CRISPR interference, or antiphage systems (Budroni et al., 2011; Oliveira et al., 2016; Doron et al., 2018; Nandi et al., 2015; Marraffini and Sontheimer, 2008) – that can act as a defense against the uptake of foreign DNA (mechanistic barriers) (Thomas and Nielsen, 2005; Eggleston et al., 1997). Finally, the effect that HGT has on the fitness of the recipient cell in a given selective environment (adaptive barrier) will determine if the recombinant genotype survives for subsequent generations (Sheppard et al., 2018; Zhu et al., 2001).
Figure 1. Barriers to horizontal gene transfer (HGT) in bacteria.
A series of barriers must be surmounted for DNA to transmit from one species to another. These are broadly defined in three categories. At a given time, alignment of holes in successive barriers is necessary for HGT to occur. Here, we focus on ecological barriers that are influenced by multiple factors that reflect the physical isolation of bacteria in separate niches.
Understanding how ecology maintains, and potentially confines, distinct strains and species has become increasingly important in the light of global challenges such as the emergence and spread of zoonotic pathogens (Boni et al., 2020). A typical approach to investigating this is to consider spillover of particular strains or clones from one host to another (clonal transmission). This is an important phenomenon and may be influenced by anthropogenic change, such as habitat encroachment or agricultural intensification (Mourkas et al., 2020). However, in many cases, important phenotypes, including antimicrobial resistance (AMR) (Johnson and Woodford, 2013; Schwarz and Johnson, 2016; Baker et al., 2018), can be conferred by relatively few genes. In such cases, it may be important to consider how cohabiting strains and species can potentially draw genes from a common pangenome pool (Young, 2016; McInerney et al., 2020; Vos and Eyre-Walker, 2017; Werren, 2011) and how genes, rather than clones, can transition between segregated populations (gene pool transmission). To investigate the impact of ecological segregation (ecological barriers) on this gene pool transmission, in natural populations, requires quantification of HGT among sympatric and allopatric bacteria.
Species within the genus Campylobacter are an ideal subject for considering how ecology influences the maintenance of genetically distinct species for several reasons. First, Campylobacter are a common component of the commensal gut microbiota of reptiles (Giacomelli and Piccirillo, 2014; Fitzgerald et al., 2014), birds (Griekspoor et al., 2013; Atterby et al., 2018), and mammals (Leatherbarrow et al., 2007) but, being microaerophilic, do not survive well outside of the host. This creates island populations that have some degree of ecological isolation. Second, because at least 12 species have been identified as human pathogens (Man, 2011) and C. jejuni and C. coli are among the most common global causes of bacterial gastroenteritis (Kaakoush et al., 2015), large numbers of isolate genomes have been sequenced from potential reservoir hosts as part of public health source-tracking programs (Sheppard et al., 2009a; Sheppard et al., 2009b). Third, within the genus there are species and strains that inhabit one or multiple hosts (ecological specialists and generalists; Mourkas et al., 2020; Griekspoor et al., 2013; Sheppard et al., 2011a; Dearlove et al., 2016; Sheppard et al., 2010; Sheppard et al., 2014; Woodcock et al., 2017). As a single host can simultaneously carry multiple lineages (Colles et al., 2008), possibly occupying different sub-niches within that host (Colles et al., 2015), there is potential to compare allopatric and sympatric populations. Finally, high-magnitude interspecies admixture (introgression) between C. jejuni and C. coli isolated from agricultural animals suggests that host ecology plays a role in the maintenance of species (Sheppard et al., 2013; Taylor et al., 2021; Sheppard et al., 2008; Sheppard et al., 2011b).
Here, we quantify HGT among 600 genomes from 30 Campylobacter species using a ‘chromosome painting’ approach (Thorell et al., 2017; Lawson et al., 2012; Yahara et al., 2013) to characterize shared ancestry among donor and recipient populations. Specifically, we investigate the role of ecological barriers to interspecies gene flow. By identifying recombining species pairs within the same and different hosts, we can describe interactions where co-localization enhances gene flow, quantify the impact of ecological barriers in these populations, and distinguish highly recombinogenic genes that are found in multiple genetic backgrounds. This provides information about the evolutionary forces that give rise to species and the extent to which ecological barriers maintain them as discrete entities.
Results
Host-restricted and host-generalist Campylobacter species
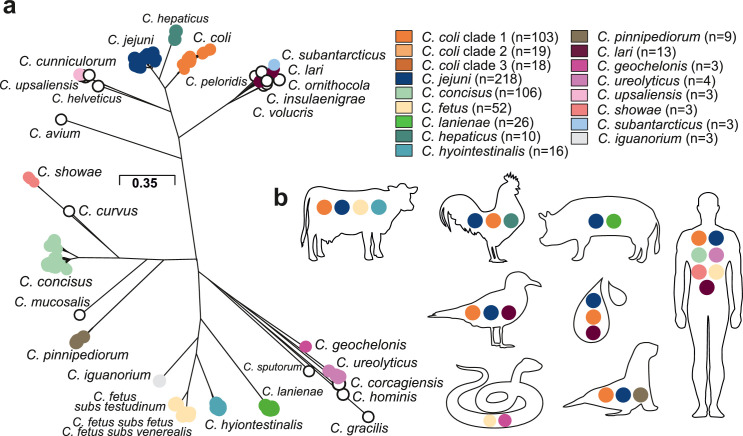
Isolate genomes were taken from publicly available databases to represent diversity within the genus Campylobacter, including environmental isolates from the closely related Arcobacter and Sulfurospirillum species, to provide phylogenetic context within the Campylobacteraceae family (Figure 2—figure supplement 1). In total, there were 631 isolates from 30 different Campylobacter species (Figure 2a) and 64 different sources, isolated from 31 different countries between 1964 and 2016 (Supplementary file 1). Among the isolates, 361 were C. jejuni and C. coli and could be classified according to 31 clonal complexes (CCs) based upon sharing four or more alleles at seven housekeeping genes defined by multilocus sequence typing (MLST) (Supplementary file 1; Dingle et al., 2001) and were representative of known diversity in both species (Mourkas et al., 2020; Sheppard et al., 2011a). The obligate human commensal and pathogen C. concisus (n = 106 isolates) comprised two genomospecies (GSI, n = 32, and GSII, n = 74), as previously described (Kirk et al., 2018; Supplementary file 1). The collection also included 52 C. fetus isolate genomes, including three subspecies: C. fetus subsp. fetus (n = 8), C. fetus subsp. venerealis (n = 23), and C. fetus subsps. testudinum (n = 21) (Supplementary file 1; Iraola et al., 2017). Two clades were observed in C. lari (Figure 2—figure supplement 2), which could correspond to previously described subspecies based on 16S rRNA sequencing (Debruyne et al., 2009).
Figure 2. Population structure and host ecology in the genus Campylobacter.
(a) Phylogenetic tree of 631 Campylobacter isolates from 30 species reconstructed using a gene-by-gene concatenated alignment of 820 core genes (shared by >95% of isolates) and an approximation of the maximum-likelihood (ML) algorithm implemented in RAxML. The species name is indicated adjacent to the associated sequence cluster. The scale bar indicates the estimated number of substitutions per site. (b) Isolation source of Campylobacter species with n ≥ 3 isolates.
Figure 2—figure supplement 1. Population structure of the Campylobacteraceae family.
Figure 2—figure supplement 2. Core genome species trees.
Figure 2—figure supplement 3. Overview of host associations of Campylobacter species.
Figure 2—figure supplement 4. Core genome species trees.
A maximum-likelihood phylogeny of the Campylobacter genus was reconstructed on a gene-by-gene concatenated sequence alignment of 820 gene families shared by >95% of all isolates, with a core genome of 903,753 base pairs (Figure 2a). The phylogeny included species that appear to be restricted to one host or environment, including: C. iguanorium (Gilbert et al., 2015) and C. geochelonis (Piccirillo et al., 2016) (reptiles); C. lanienae (Logan et al., 2000) (pigs); C. hepaticus (Van et al., 2016) (chicken liver); the C. lari group (Miller et al., 2014) (marine birds and environment); C. pinnipediorum (Gilbert et al., 2017) (seals) species - most of which were discovered recently (Figure 2—figure supplement 3). There was no evidence that phylogeography was reflected in the observed population structure for Campylobacter isolates from multiple hosts and countries (Figure 2—figure supplement 4). This is unsurprising as it is well known that host-associated genetic variation transcends phylogeographic structuring in Campylobacter (Sheppard et al., 2010). While some low-level local gene flow can be identified within a given country (Pascoe et al., 2017), this is vastly outweighed by recombination within particular host niches (Sheppard et al., 2014), particularly in small isolate collections such as those for some of the species in this study.
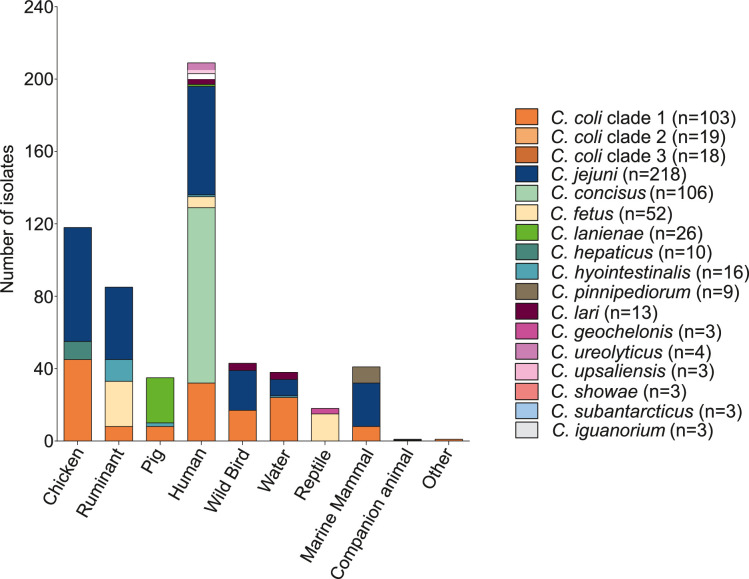
Host-restricted species had lower diversity possibly linked to low sample numbers, with C. hepaticus having the lowest diversity (Figure 2—figure supplement 2) with 8/10 genomes associated with isolates from the same outbreak (Van et al., 2016). For other species, there was evidence of a broad host range (ecological generalists) (Figure 2b). For example, highly structured C. jejuni and C. coli isolates were sampled from seven and six host sources, respectively (Figure 2—figure supplement 2, Figure 2—figure supplement 3, Supplementary file 1). For C. fetus, there was distinct separation between mammal-associated C. fetus subsp. fetus and C. fetus subsp. venerealis and reptile-associated C. fetus subsp. testudinum (Figure 2—figure supplement 2) as previously described (Iraola et al., 2017). Unsurprisingly, a large proportion of the isolates in this study were from humans, likely reflecting intensive sampling. C. jejuni (27.52%; n = 60/218), C. coli (14.68%; n = 32/218), and C. concisus (44.5%; n = 97/218) were all common among human clinical samples. However, less common species were also present, with nearly half of all Campylobacter species (44.83%, n = 13/29) isolated from humans at least once (Figure 2b, Supplementary file 1). Agricultural animals were also a common source accounting for more than 1/3 of the isolates (38.35%; 242/631), with 10/30 Campylobacter species isolated from more than one source (Figure 2b, Supplementary file 1).
Evidence of interspecies recombination in the core and accessory genome
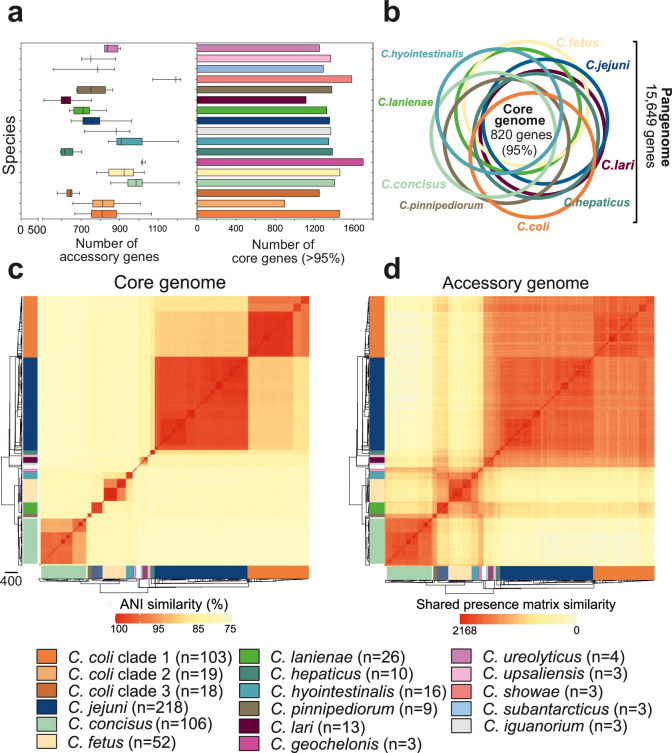
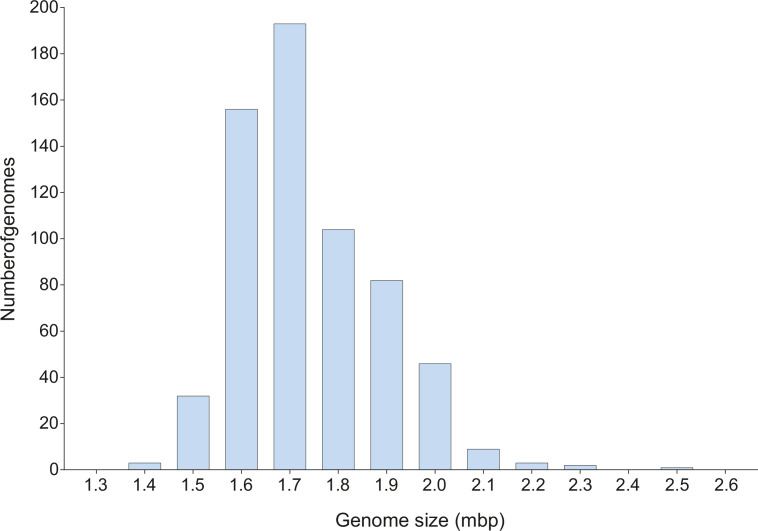
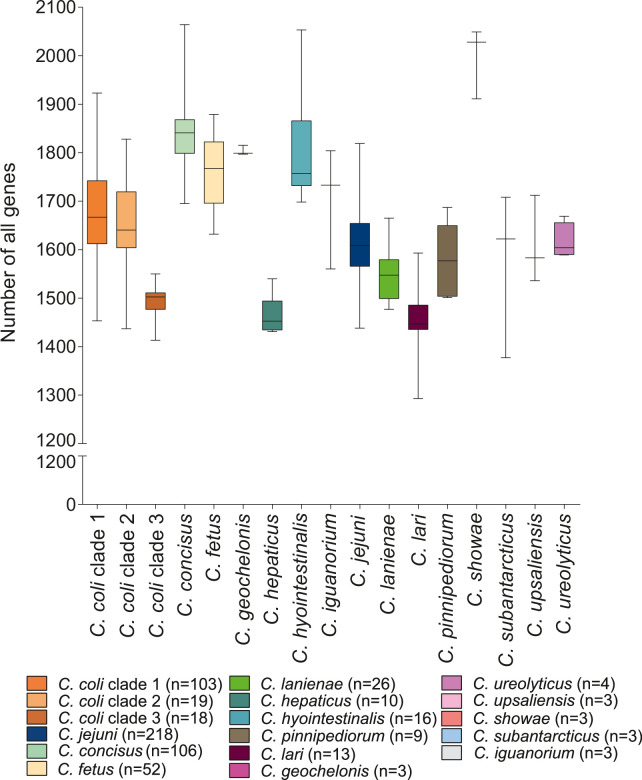
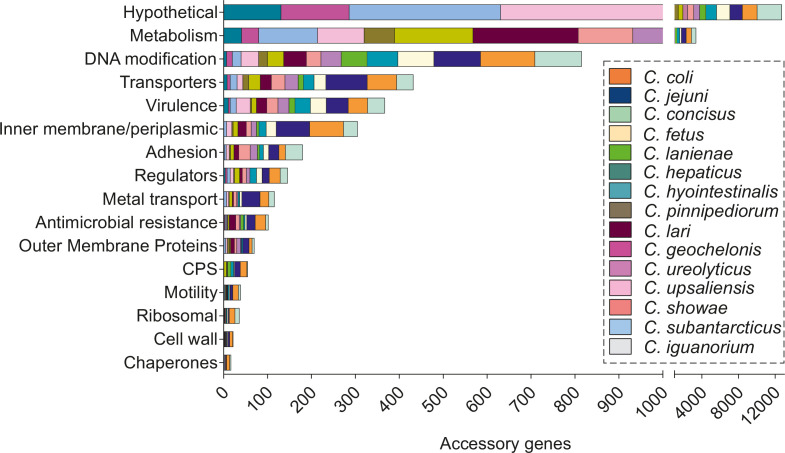
Genome size varied between 1.40 and 2.51 Mb (Figure 3—figure supplement 1) (mean 1.73), and the number of genes (per isolate) ranged from 1,293 to 2,170 (mean 1,675) (Figure 3—figure supplement 2). The pangenome for the genus comprised 15,649 unique genes, found in at least one of the 631 isolates (Figure 3—source data 1), with 820 genes (5.24% of the pangenome) shared by >95% of all isolates (core genome), across 30 species (Figure 3—source data 1). We excluded species with fewer than three isolates in subsequent analysis. For the remaining 15 species, the core genome ranged in size from 1,116 genes in C. lari to 1,700 in C. geochelonis (Figure 3a, right panel, Figure 3—source data 1). Differences were also noted in the size of accessory genomes, with C. concisus (mean: 981 genes), C. hyointestinalis (mean: 946 genes), C. showae (mean: 1,160 genes), C. geochelonis (mean: 1,021 genes), and C. fetus (mean: 912 genes) containing the highest average number of accessory genes (Figure 3a, left panel, Figure 3—source data 1). Functional annotation of all 14,829 accessory genes showed that 71% (10,561) encoded hypothetical proteins of unknown function due to the lack of homology with well-characterized genes (Figure 3—figure supplement 3; Pascoe et al., 2019). Remaining genes were related to metabolism, DNA modification, transporters, virulence, inner membrane/periplasmic, adhesion, regulators, metal transport, and AMR (Figure 3—figure supplement 3).
Figure 3. Core and accessory genome variation in the genus Campylobacter.
(a) Overall distribution of the total number of accessory genes (left) and core genes (right) per isolate for each Campylobacter species (where n ≥ 3 isolates). The number of accessory genes is shown as boxplots (min to max). (b) Venn diagram of pangenomes among different Campylobacter species (n ≥ 9). The number of core genes shared by all species is illustrated in the center. (c) Pairwise average nucleotide identity (ANI) comparison calculated for all 631 Campylobacter isolates based upon 820 core genes shared by >95% of isolates. ANI values < 75% are not calculated by FastANI (Jain et al., 2018). (d) Pairwise accessory genome similarity based upon gene presence or absence at 2,168 non-core loci. The heatmap coloring ranges from yellow (minimum) to red (maximum). The matrices are ordered according to the phylogenetic tree presented in Figure 2a. Different colors correspond to Campylobacter species with ≥3 isolates.
Figure 3—figure supplement 1. Genome size variation of the Campylobacter genus.
Figure 3—figure supplement 2. Gene variation in the genus Campylobacter.
Figure 3—figure supplement 3. Accessory gene function in all main Campylobacter species.
Figure 3—figure supplement 4. Core genome allelic variation and the effect of recombination.
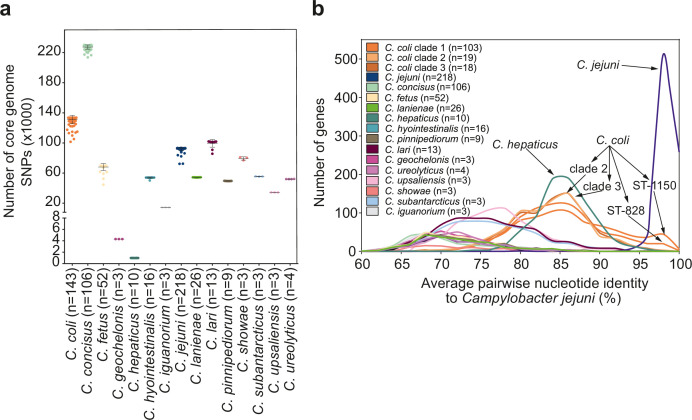
To further understand genetic differentiation within and between species, we generated genus-wide similarity matrices for the core and accessory genomes (Figure 3c and d, Figure 3—source data 1). For the core genome, pairwise average nucleotide identity (ANI) was calculated for shared genes in all possible genome pairs (Figure 3—source data 1) using FastANI (Jain et al., 2018). On average, isolates of the same species shared >95% similarity (Figure 3—source data 1), with decreasing genetic similarity (between 85 and 90%) over greater phylogenetic distances. The number of core genome SNPs ranged from 983 to 230,264 for the 15 Campylobacter species with ≥3 isolates in our dataset, with C. coli and C. concisus having the greatest mean SNP numbers (Figure 3—figure supplement 4a), indicating considerable diversity within these species. In contrast, C. hepaticus and C. geochelonis had low mean SNP numbers with 986 and 4,310, respectively. This is likely related to low sample numbers with isolates either sampled in close proximity (Piccirillo et al., 2016) or from a single outbreak (Van et al., 2016).
The core genome similarity matrix provided initial evidence of interspecies gene flow (introgression). This can be observed as elevated nucleotide identity between C. jejuni and clade 1 C. coli (Figure 3—source data 1), consistent with previous studies (Sheppard et al., 2013; Sheppard et al., 2008; Sheppard et al., 2011b). Further evidence of introgression came from pairwise ANI comparison of genus-wide core genes, in all isolates of the 15 major Campylobacter species, to the C. jejuni genome (Figure 3—figure supplement 4b). In the absence of gene flow, isolates from the two species should have an approximately unimodal ANI distribution reflecting accumulation of mutations throughout the genome. This was largely the case, but for some species, low nucleotide divergence suggested recent recombination with C. jejuni. There was also evidence of interspecies accessory genome recombination. Presence/absence patterns in the accessory genome matrix show considerable accessory gene sharing among several species that was inconsistent with the phylogeny (Figure 3—source data 1). This is well illustrated in C. lanienae where much of the accessory genome was shared with other Campylobacter species (Figure 3—source data 1).
Enhanced interspecies recombination among cohabiting species
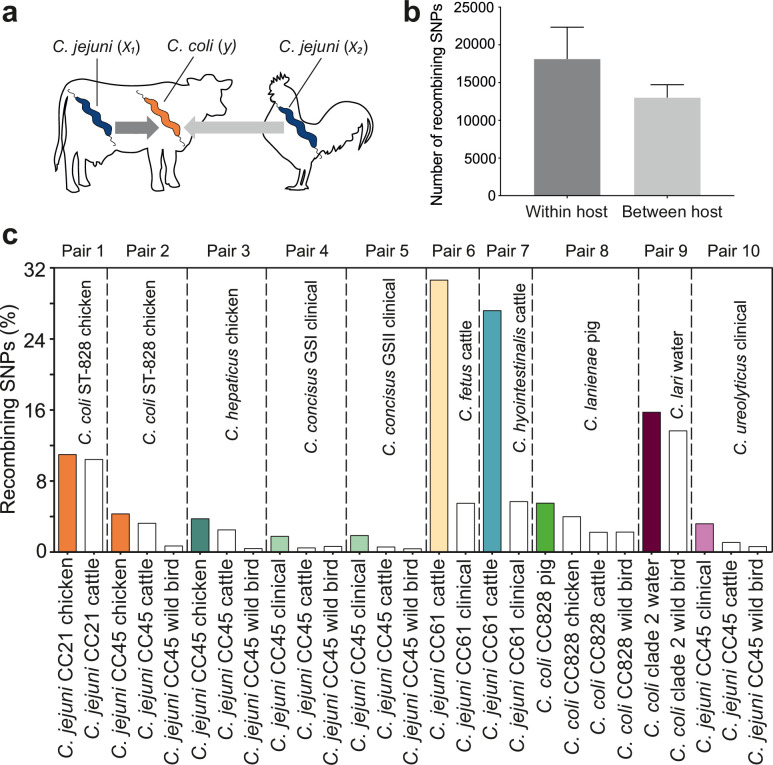
For Campylobacter inhabiting different host species, there is a physical barrier to HGT. However, when there is niche overlap, interspecies recombination can occur, for example, between C. jejuni and C. coli inhabiting livestock (Sheppard et al., 2011a; Sheppard et al., 2013; Sheppard et al., 2008). To understand the extent to which inhabiting different hosts impedes interspecies gene flow, we quantified recombination among Campylobacter species where isolates originated from same host (x1, y) and different hosts (x2, y) (Figure 4a).
Figure 4. Elevated within-host interspecies recombination and donor–recipient comparisons.
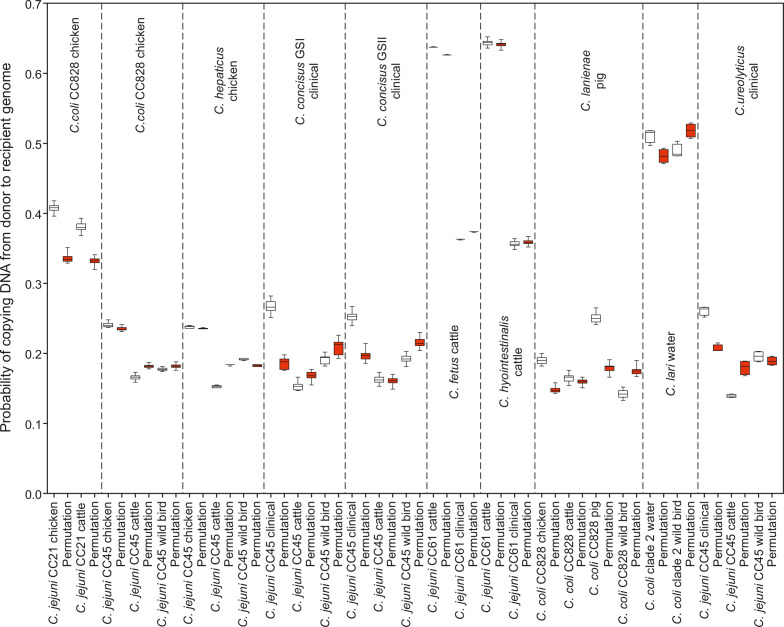
(a) A hypothesis depicting the relationships between Campylobacter species, C. jejuni (x1, x2) and C. coli (y), when found in the same or in different hosts. (b) Number of recombining SNPs within and between host as inferred by chromosome painting analysis for all donor–recipient species comparisons. The error bar represents the standard error of the mean (SEM). (c) The figure shows the number of donated SNPs in 10 donor–recipient pair species comparisons. The proportion (%) of recombining SNPs with >90% probability of copying from a donor to a recipient genome is illustrated on the y-axis. All donor groups are shows in the x-axis. All colored boxes correspond to comparison where donors and recipients are found in the same host.
Figure 4—figure supplement 1. Probability of the recipient genomes sharing DNA with each donor groups is illustrated as box whiskers (white) for every donor–recipient comparison for all 10 pairs that supported our hypothesis.
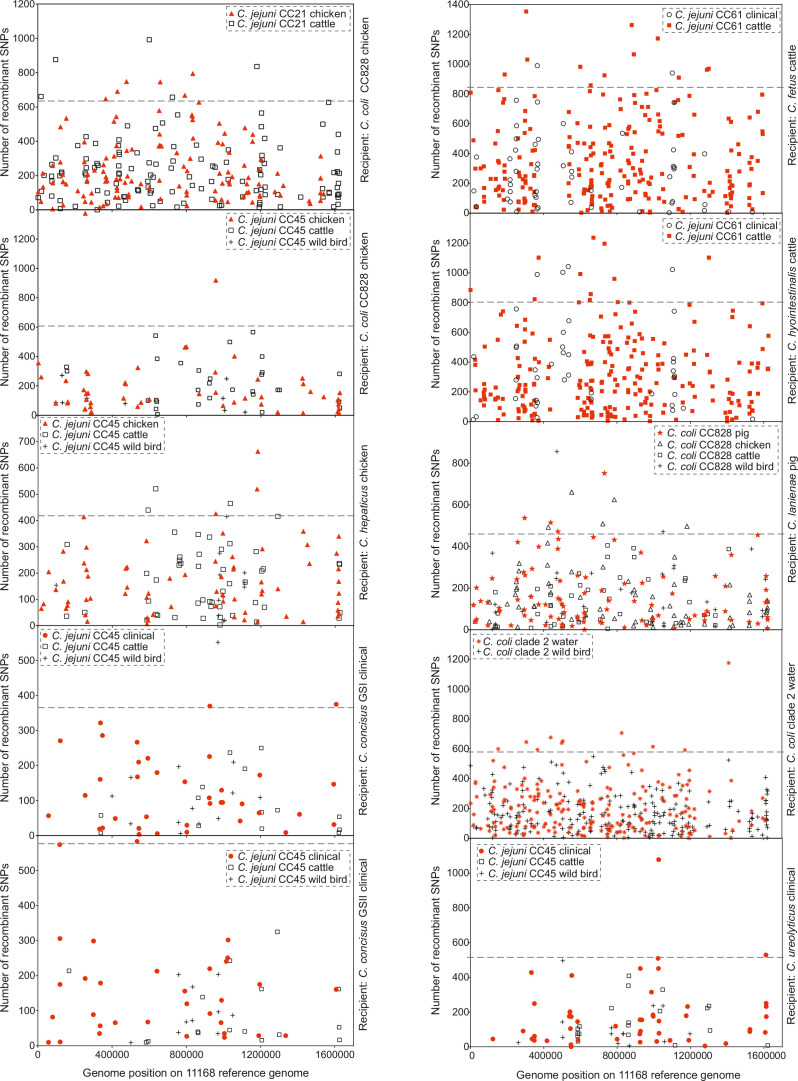
Figure 4—figure supplement 2. Genome position of genes containing recombining SNPs.
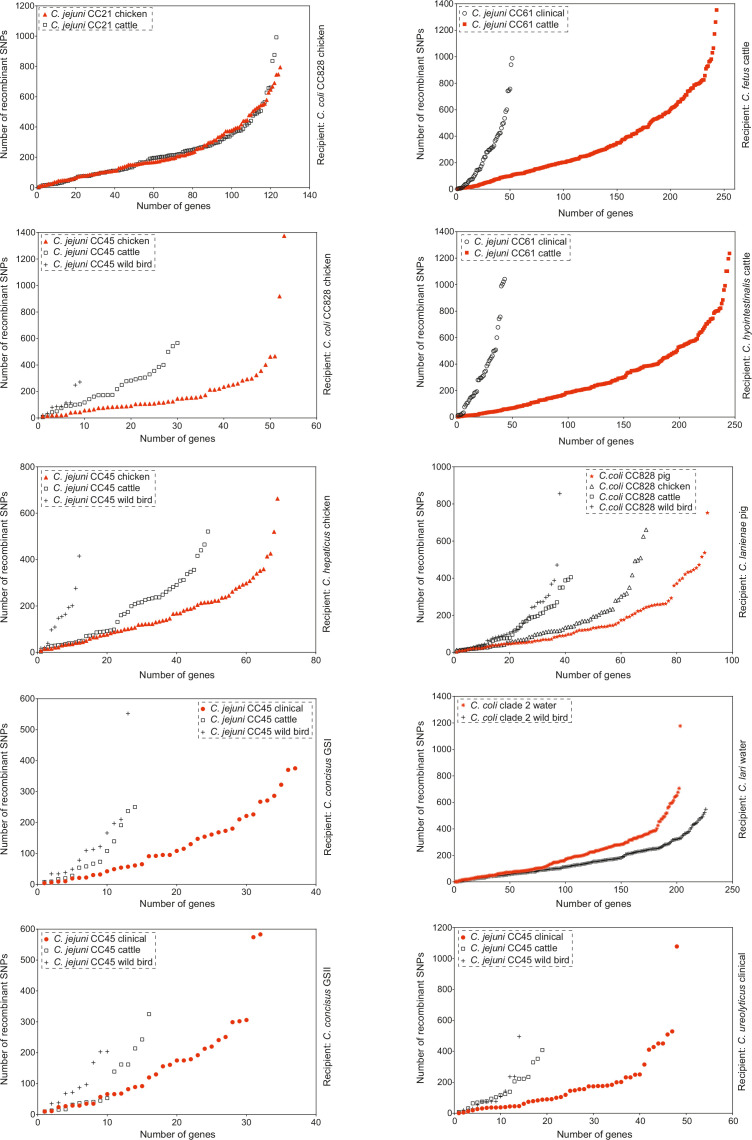
Figure 4—figure supplement 3. Genes ranked in ascending order of the number of recombining SNPs they contain as inferred by chromosome painting analysis for all 10 species comparisons.
ChromoPainterV2 software was used to infer tracts of DNA donated from multiple donor groups, belonging to the same CC but isolated from different hosts to recipient groups (Materials and methods). Among 27 combinations of multiple donor groups and recipient groups, overall, there were more recombining SNPs within hosts than between hosts (Figure 4b), and for 10/27 species pairs there was evidence of enhanced within-species recombination (x1 → y > x2 → y; Figure 4c). To assess the robustness of the analysis, we included the effect of randomization and repeated the analysis by assigning random hosts for every strain (Figure 4—figure supplement 1). In the 10 pair species comparisons where x1 → y > x2 → y, we detected 174,594 within-host recombining SNPs (mapped to 473 genes; 28.8% of NCTC11168 genes) and 109,564 between-host recombining SNPs (mapped to 395 genes; 24.05% of NCTC11168 genes). From the 473 within-host recombining genes, 45 genes contained the highest number (>95th percentile) of recombining SNPs (Figure 4—figure supplement 2, Figure 4—figure supplement 3, Supplementary file 2). These genes have diverse inferred functions including metabolism, cell wall biogenesis, DNA modification, transcription, and translation (Supplementary file 2).
Interspecies recombination was observed for isolates sampled from chickens between generalist lineages CC21 and CC45 (donors; C. jejuni) and generalist CC828 (recipient; C. coli). These lineages appear to have high recombination to mutation (r/m) ratio as inferred by ClonalFrameML (Supplementary file 3). DNA from generalist C. jejuni CC45 was introduced into three Campylobacter species, including C. hepaticus (chicken), C. concisus GSI and GSII (clinical), and C. ureolyticus (clinical) (Figure 4c, Figure 4—figure supplement 2, Figure 4—figure supplement 3 Supplementary file 4). CC 45 had the highest r/m ratio from all other lineages or species involved in the comparisons (Supplementary file 3). There was increased recombination in genomes sampled from cattle between C. jejuni CC61 (donor; C. jejuni) and C. fetus and C. hyointestinalis (recipients) with 71.75% of all within-host recombining SNPs from all 10 comparisons detected in these two pairs (Figure 4c, Figure 4—figure supplement 2, Figure 4—figure supplement 3, Supplementary file 4). Agricultural-associated C. jejuni CC61 and C. fetus subsp. venerealis involved in these comparisons were among the lineages and subspecies with the highest r/m ratios (Supplementary file 3). The cattle-associated CC61 has previously been described as highly recombinant and has been associated with rapid clonal expansion and adaptation in cattle (Mourkas et al., 2020).
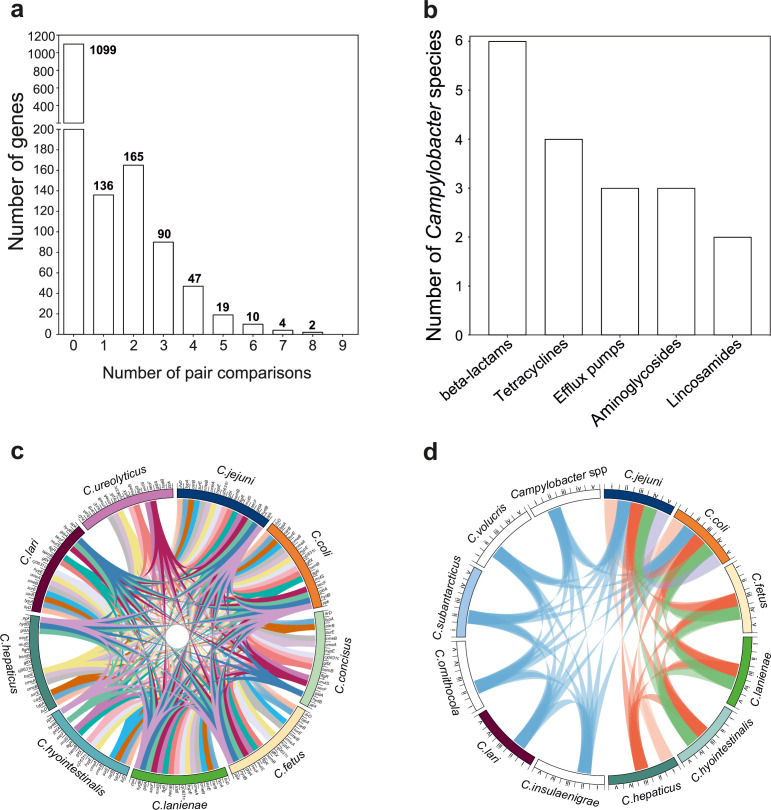
The within-host mobilome
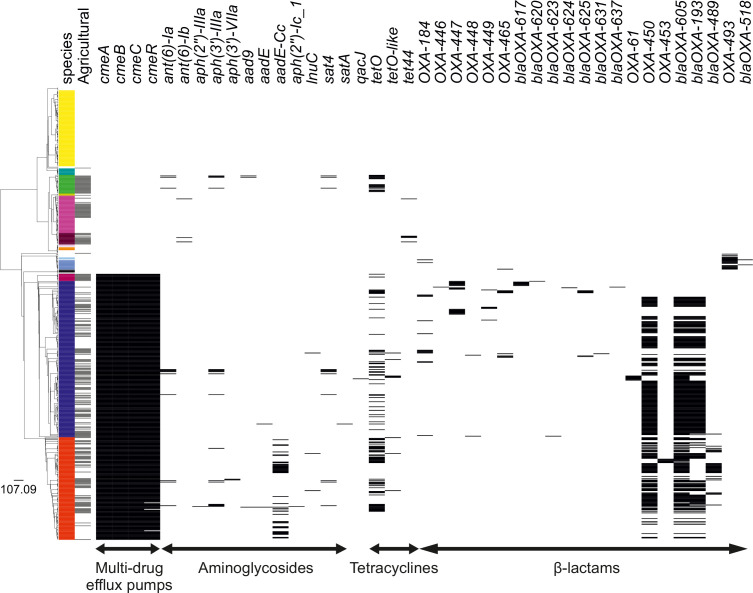
Bacteria inhabiting the same niche may benefit from functionality conferred by similar gene combinations. Recombination can promote the dissemination of adaptive genetic elements among different bacterial species. Therefore, we postulated that the genes that recombine most among species (>95th percentile) will include those that are potentially beneficial in multiple genetic backgrounds. To investigate this, we quantified mobility within the genome identifying recombining SNPs found in more than one species comparison (Figure 5a). These SNPs mapped to 337 genes (20.52% of the NCTC11168 genes; 2.15% of the pangenome) (Figure 5a, Supplementary file 5). We found that 32 of those genes (9.49%) have also been found on plasmids (Supplementary file 5). A total of 16 genes showed elevated within-host interspecies recombination in more than five species pairs (Figure 5c, Supplementary file 5). Genes included cmeA and cmeB, which are part of the predominant efflux pump CmeABC system in Campylobacter. Sequence variation in the drug-binding pocket of the cmeB gene has been linked to increased efflux function leading to resistance to multiple drugs (Yao et al., 2016). Many of the same antimicrobial classes are used in human and veterinary medicine, and this may be linked to selection for AMR Campylobacter, which are commonly isolated from livestock (Livermore, 2007). To investigate this further, we compared the genomes of all 631 isolates in our dataset to 8,762 known antibiotic resistance genes from the Comprehensive Antibiotic Resistance Database (CARD) (Jia et al., 2017), ResFinder (Zankari et al., 2012), and the National Center for Biotechnology Information (NCBI) databases. Homology (>75%) was found for 42 AMR determinants associated with multidrug efflux pumps, aminoglycosides, tetracyclines, and β-lactams (Figure 5b, Figure 5—figure supplement 1, Figure 5—source data 1). Species that contained >40% isolates from livestock, including C. jejuni, C. coli, C. lanienae, C. hepaticus, C. hyointestinalis, and C. fetus, contained far more AMR determinants (Figure 5d, Figure 5—figure supplement 1, Figure 5—source data 1). AMR genes are often collocated in the genome (Mourkas et al., 2019), and our analysis revealed several gene clusters (Figure 5—figure supplement 2) that have been described in previous studies (Mourkas et al., 2019; Abril et al., 2010). These findings are consistent with HGT-mediated circulation of AMR genes among different Campylobacter species and support the hypotheses that ecology drives gene pool transmission (Sheppard et al., 2018; Mourkas et al., 2019).
Figure 5. The mobilome of the Campylobacter genus.
(a) The graph illustrates the proportion of recombining genes in 10 different species comparisons. The number of species pairs in which the gene was found to recombine is shown on the x-axis, and the number of genes in each category is given on the y-axis. The exact number of genes found in each group comparison is shown on the top of each box. (b) Number of Campylobacter species harboring antimicrobial resistance (AMR) genes that belong to efflux pumps and four different antibiotic classes that are shown on the x-axis. (c) The circos plot indicates the 16 genes involved in recombination in >5 donor–recipient pair species comparisons. Gene matches are indicated by joining lines, colored differently for each gene. Gene names are shown around the perimeter for each Campylobacter species. (d) The circos plot indicates the sharing of AMR genes associated with efflux pumps and four antibiotic classes among Campylobacter species. Presence of at least one gene (not necessarily the same gene) conferring resistance to a specific antibiotic class is indicated by joining lines, colored differently for each drug class. Efflux pumps (i), β-lactams (ii), tetracyclines (iii), aminoglycosides (iv), and lincosamides (v) are shown around the perimeter for each Campylobacter species.
Figure 5—figure supplement 1. Presence of antimicrobial resistance (AMR) genes in the Campylobacter genus.
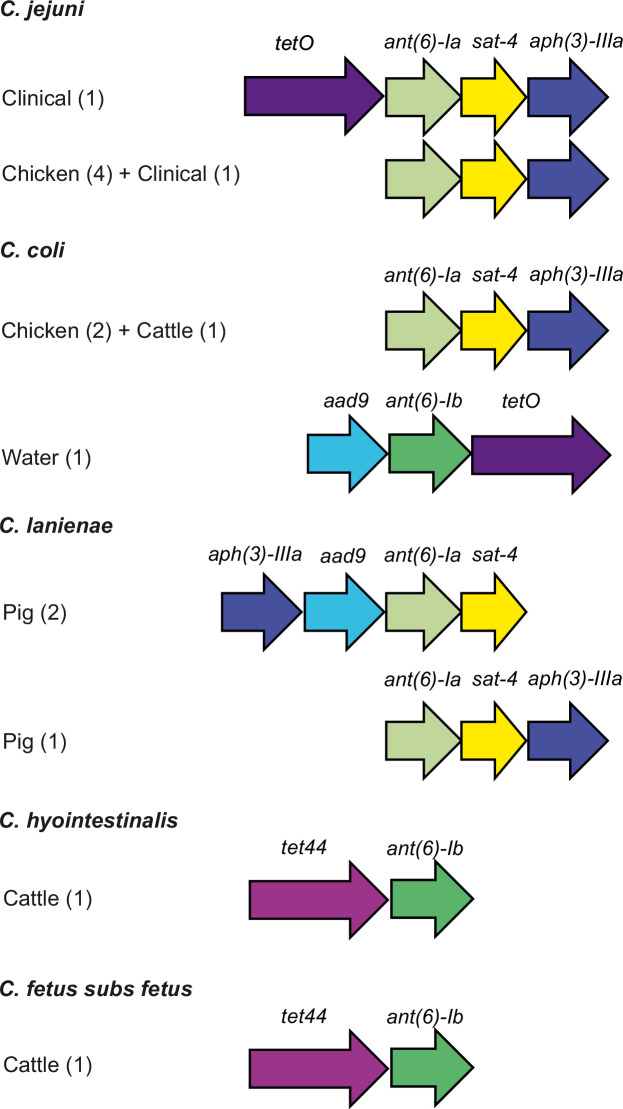
Figure 5—figure supplement 2. Genetic organization of antimicrobial resistance (AMR) genes in Campylobacter.
Campylobacter host transmission and virulence have been linked with biofilm formation and changes in surface polysaccharides (Szymanski et al., 2003; McLennan et al., 2008). The carB gene showed elevated within-host interspecies recombination in eight species pair comparisons (Figure 5c, Supplementary file 5). This gene encodes a carbamoylphosphate synthase that has been associated with biosynthesis of substrates for many polysaccharides and is known to contain transposon insertion sites upstream of its genomic position (McLennan et al., 2008). Other genes with elevated within-host interspecies transfer (>7 species pairs) included typA (Figure 5c, Supplementary file 5), a translator regulator for GTPase and gltX (Figure 5c), a glutamate-tRNA ligase, promoting survival under stress conditions (Margus et al., 2007; Semanjski et al., 2018). Other genes included gidA and hydB associated with virulence (Mikheil et al., 2012) and hydrogenase enzyme activity (respiratory pathway in C. concisus, Benoit and Maier, 2018), respectively. By considering genes that overcome barriers to interspecies recombination and establish in multiple new genetic backgrounds, it may be possible to infer important phenotypes that allow bacteria to adapt to different hosts and environments.
Discussion
Phylogenetic reconstruction of the genus Campylobacter revealed a highly structured population. Distinct core genome clustering largely supported known classification for species, subspecies (C. fetus, Iraola et al., 2017), genomospecies (C. concisus, Kirk et al., 2018), and clades (C. coli Sheppard et al., 2008). Also consistent with previous studies, certain species are principally associated with a specific host niche. For example, C. fetus subsp. testudinum, C. iguanorium, and C. geochelonis were only sampled from reptile species, and C. pinnipediorum was only sampled from seals. However, for several species there was clear evidence for host generalism, including C. jejuni, C. coli, and C. lari, all of which were sampled from multiple hosts (Griekspoor et al., 2013; Cody et al., 2015). It is clear that the hosts with the greatest diversity of Campylobacter species were agricultural animals (and humans) (Figure 2—figure supplement 3). While this undoubtedly reflects oversampling of these sources to some extent, the cohabitation of species in the same host niche potentially provides opportunities for interspecies HGT.
Initial evidence of interspecies gene flow came from comparison of ANI and the accessory genome gene presence/absence for all isolates. In each case, patterns of genetic similarity largely mirrored the phylogeny. However, consistent with previous studies (Sheppard et al., 2013), there was clear evidence of elevated homologous and non-homologous recombination between some species. For example, core genome ANI was higher between C. jejuni and C. coli clade 1 compared to other C. coli clades (Figure 3—source data 1). The evidence for non-homologous gene sharing was even more striking with accessory genome sharing across considerable genetic distances (Figure 3—source data 1), exemplified by C. lanienae, which shares accessory genes with most other Campylobacter species.
To quantify the extent to which ecological barriers influenced interspecies gene flow, it was necessary to focus on donor–recipient species pairs where there was evidence of elevated HGT in the same (sympatry) compared to different (allopatry) hosts. Perhaps unsurprisingly, this was not the case for all species comparisons. Interacting factors could lead to genetic isolation even when species inhabit the same host. First, rather than being a single niche, the host represents a collection of subniches with varying degrees of differentiation. For example, gut-associated bacteria in the same intestinal tract have been shown to occupy different microniches (Hayashi et al., 2005) and more striking segregation may be expected between C. hepaticus inhabiting the liver in poultry (Van et al., 2016) and gut-dwelling C. jejuni and C. coli in the same host. Second, there is potential for the resident microbiota to influence the colonization potential of different Campylobacter species and therefore the opportunity for genetic exchange, for example, through succession (Lu et al., 2003) and inhibition of transient species by residents, as seen in some other bacteria in humans (Stecher et al., 2010; van Elsas et al., 2012; Nowrouzian et al., 2005).
Continued exposition of the microecology of subniches is important, but for 10 species comparisons, there was clear evidence of enhanced within-host gene flow allowing quantitative analysis of ecological barriers to gene flow. Specifically, there was on average a three-fold increase in recombination among species pairs inhabiting the same host. In some cases, this was greater, with 5–6 times more recombination among cohabiting species C. jejuni and C. hyointestinalis/C. fetus in cattle. In absolute terms, this equates to approximately 30% of all recorded SNPs in the recipient species being the result of introgression. To place this in context, if greater than half (51%) of the recorded SNPs resulted from interspecies recombination then the forces of species convergence would be greater than those that maintain distinct species. If maintained over time, these relative rates could lead to progressive genetic convergence unless countered by strong genome-wide natural selection against introgressed DNA.
Quantitative SNP-based comparisons clearly ignore one very important factor. Specifically, that recombined genes that do not reduce the fitness of the recipient genome (provide an adaptive advantage) will remain in the population while others will be purged through natural selection. Therefore, by identifying genomic hotspots of recombination and the putative function of genes that recombine between species, it is possible to understand more about microniche segregation and the host-adapted gene pool. Of the 35 genes with evidence of enhanced within-host HGT in ≥5 species pairs, several were linked to functions associated with proliferation in, and exploitation of, the host. For example, the carB gene, encoding the large subunit of carbamoylphosphatase associated with polysaccharide biosynthesis, recombined in eight cohabiting species pairs and is potentially linked to enhanced virulence and growth (McLennan et al., 2008). In addition, other highly mobile genes, including typA and gltX, are associated with survival and proliferation in stress conditions (Margus et al., 2007; Semanjski et al., 2018), and hydB is linked to NiFe hydrogenase and nickel uptake that is essential for the survival of C. jejuni in the gut of birds and mammals (Howlett et al., 2012).
Some genes showed evidence of elevated recombination in a specific host species. For example, the glmS and napA genes in cohabiting Campylobacter species in cattle. In many bacteria, analogs of glmS have multiple downstream integration-specific sites (Tn7) (Choi and Kim, 2009), which may explain the mobility of this gene. Explaining the mobility of napA is less straightforward, but this gene is known to encode a nitrate reductase in Campylobacter (Pittman et al., 2007) in microaerobic conditions, which may be ecologically significant as the accumulation of nitrate in slurry, straw, and drainage water can be potentially toxic to livestock mammals (Alexander et al., 2009).
Factors such as host physiology, diet, and metabolism undoubtedly impose selection pressures upon resident bacteria, and the horizontal acquisition of genes provides a possible vehicle for adaptation. However, the widespread use of antimicrobials by humans, pets, and livestock production (Teuber, 2001; Price et al., 2015) provides another major ecological barrier to niche colonization. We found that gyrA was among the most recombinogenic genes in Campylobacter in chickens. This is important as a single mutation in this gene is known to confer resistance to ciprofloxacin (Luo et al., 2003). While the rising trend in fluoroquinolones resistance in Campylobacter from humans and livestock (Sproston et al., 2018) may result from spontaneous independent mutations, it is likely that it is accelerated by HGT. However, there is currently no clear evidence for the transfer of resistant versions of gyrA. Interspecies recombination of AMR genes has been observed between C. jejuni and C. coli isolates from multiple sources including livestock, human, and sewage (Mourkas et al., 2019). Consistent with this, we found AMR genes present in strains from 12 Campylobacter species in multiple hosts (Figure 5—figure supplement 2). In some cases, strains from phylogenetically closely related species (C. fetus and C. hyointestinalis) isolated from cattle shared the same AMR gene cluster (tet44 and ant(6)-Ib) described before in C. fetus subsp. fetus (Abril et al., 2010), indicating the circulation of colocalized AMR genes among related species and host niche gene pools. Strikingly, the efflux pump genes cmeA and cmeB, associated with multidrug resistance (MDR), were highly mobile among Campylobacter species with evidence of elevated within-host interspecies recombination in >7 species pairs. Furthermore, the gltX gene, which when phosphorylated by protein kinases promotes MDR (Semanjski et al., 2018), was also among the most introgressed genes. While a deeper understanding of gene interactions, epistasis, and epigenetics would be needed to prove that the lateral acquisition of AMR genes promotes niche adaptation, these data do suggest that HGT may facilitate colonization of antimicrobial-rich host environments, potentially favoring the spread of genes into multiple genetic backgrounds.
In conclusion, we show that species within the genus Campylobacter include those that are host restricted as well as host generalists. When species cohabit in the same host, ecological barriers to recombination can be perforated, leading to considerable introgression between species. While the magnitude of introgession varies, potentially reflecting microniche structure within the host, there is clear evidence that ecology is important in maintaining genetically distinct species. This parallels evolution in some interbreeding eukaryotes, such as Darwin’s Finches, where fluctuating environmental conditions can change the selection pressures acting on species inhabiting distinct niches, potentially favoring hybrids (Mallet, 2007; Grant and Grant, 1992). Consistent with this, the host landscape is changing for Campylobacter, with intensively reared livestock now constituting 60–70% of bird and mammal biomass on earth, respectively (Bar-On et al., 2018). This creates opportunities for species to be brought together in new adaptive landscapes and for genes to be tested in multiple genetic backgrounds. By understanding the ecology of niche segregation and the genetics of bacterial adaptation, we can hope to improve strategies and interventions to reduce the risk of zoonotic transmission and the spread of problematic genes among species.
Materials and methods
Isolate genomes
A total of 631 Campylobacter, 17 Arcobacter, 7 Sulfurospirillum, and 5 Helicobacter genomes were assembled from previously published datasets (Supplementary file 1). Isolates were sampled from clinical cases of campylobacteriosis and feces of chickens, ruminants, wild birds, wild mammals, pets, and environmental sources. Genomes and related metadata were uploaded and archived in the BIGS database (Sheppard et al., 2012). Quality control was performed based on the genome size, number of contigs, and N50 and N95 contig length using the integrated tools in BIGS database. All assembled contigs were further screened for contamination and completeness using CheckM (Parks et al., 2015; Supplementary file 1). All assembled genomes can be downloaded from FigShare (doi: 10.6084/m9.figshare.15061017). Comparative genomics analyses focused on the Campylobacter genomes representing 30 species including C. avium (n = 1); C. coli (n = 143); C. concisus (n = 106); C. corcagiensis (n = 1); C. cuniculorum (n = 2); C. curvus (n = 2); C. fetus (n = 52); C. geochelonis (n = 3); C. gracilis (n = 2); C. helveticus (n = 1); C. hepaticus (n = 10); C. hominis (n = 1); C. hyointestinalis (n = 16); C. iguanorium (n = 3); C. insulaenigrae (n = 1); C. jejuni (n = 218); C. lanienae (n = 26); C. lari (n = 13); C. mucosalis (n = 1); C. ornithocola (n = 1); C. peloridis (n = 1); C. pinnipediorum (n = 9); C. rectus (n = 1); C. showae (n = 3); C. sputorum (n = 1); C. subantarcticus (n = 3); C. upsaliensis (n = 3); C. ureolyticus (n = 4); C. volucris (n = 2); and Campylobacter sp. (n = 1) (Supplementary file 1). Genomes belonging to C. jejuni and C. coli species were selected to represent a wide range of hosts, sequence types, and CCs and reflect the known population structure for these two species. For other Campylobacter species, all genomes that were publicly available at the time of this study were included in the analysis (Supplementary file 1).
Pangenome characterization and phylogenetic analysis
Sequence data were analyzed using PIRATE, a fast and scalable pangenomics tool that allows for ortholog gene clustering in divergent bacterial species (Bayliss et al., 2019). Genomes were annotated in Prokka (Seemann, 2014) using a genus database comprising well-annotated C. jejuni strains NCTC11168, 81116, 81-176 and M1, and plasmids pTet and pVir in addition to the already existing databases used by Prokka (Seemann, 2014). Briefly, annotated genomes were used as input for PIRATE. Nonredundant representative sequences were produced using CD-HIT, and the longest sequence was used as a reference for sequence similarity interrogation using BLAST/DIAMOND. Gene orthologs were defined as ‘gene families’ and were clustered in different MCL thresholds, from 10 to 98% sequence identity (10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 98). Higher MCL thresholds were used to identify allelic variation within different loci. An inflation value of 4 was used to increase the granularity of MCL clustering between gene families. BLAST high-scoring pairs with a reciprocal minimum length of 90% of the query/subject sequence were excluded from MCL clustering to reduce the number of spurious associations between distantly related or conserved genes (Sahl et al., 2014). This information was used to generate gene presence/absence and allelic variation matrices. A core gene-by-gene multiple sequence alignment (Sheppard et al., 2012) was generated using MAFFT (Katoh et al., 2002) comprising genes shared >95% of isolates. Phylogenetic trees, based on core gene-by-gene alignments, were reconstructed using the maximum-likelihood algorithm implemented in RAxML v8.2.11 (Stamatakis, 2014) with GTRGAMMA as substitution model.
Quantifying core and accessory genome variation
The degree of genetic differentiation between species was investigated gene-by-gene as in previous studies (Sheppard et al., 2013; Didelot et al., 2007) by calculating the ANI of all 631 Campylobacter genomes using FastANI v.1.0 (Jain et al., 2018). The analysis generated a lower triangular matrix with the lowest ANI value at 75% (as computed by FastANI). A comparable gene presence/absence matrix was produced using PIRATE and was further used to generate a heatmap of accessory genome similarity based upon gene presence or absence. Subsequently, all Campylobacter genomes were screened for the presence of AMR genes against the CARD (Jia et al., 2017), ResFinder (Zankari et al., 2012), and NCBI databases. All Campylobacter genomes were further screened for the presence of phage, conjugative elements, and plasmid DNA using publicly available online databases to investigate the effect of other transfer mechanisms. First, we used the PHAge Search Tool Enhanced Release (PHASTER) (Arndt et al., 2016) to identify and annotate prophage sequences within our genomes. A total of 86% (254/297) of the genomes used in chromosome painting were found to have DNA sequence of phage origin. Second, we used Iceberg 2.0 (Liu et al., 2019) for the detection of integrative and conjugative elements, identifying 32 ICEs in 19% (56/297) of the genomes used in the chromosome painting analysis. Finally, we used MOB-suite software for clustering, reconstructing, and typing of plasmids from draft assemblies (Robertson and Nash, 2018; Robertson et al., 2020). A positive hit was defined when a gene had >75% nucleotide identity over >50% of the sequence length showing that 32 genes identified in the recombination analysis have also been located on plasmids. A gene presence/absence matrix for every AMR gene was generated for every genome. Genomes carrying AMR genes were screened to characterize the location of adjacent genes using SnapGene software (GSL Biotech; available at https://www.snapgene.com/), as previously described (Mourkas et al., 2019). The number of core SNPs was identified using SNP-sites (v2.3.2) (Page et al., 2016).
Inference of recombination
Each combination of a recipient group and multiple donor groups (belonging to the same CC but isolated from different hosts) was selected to compare the extent of interspecies recombination into the recipient genomes. Each donor group consisted of eight isolates to avoid the influence of difference in sample size on estimation of the extent of interspecies recombination. Each recipient group included at least four isolates. We excluded C. jejuni and C. coli clade 1 genomes isolated from seals and water as these most likely represent spillover events and not true host-segregated populations. Briefly, we conducted a pairwise genome alignment between reference genome NCTC11168 and one of the strains included in the donor–recipient analysis using progressiveMauve (Darling et al., 2010). This enabled the construction of positional homology alignments for all genomes regardless gene content and genome rearrangements, which were then combined into a multiple whole-genome alignment, as previously described (Yahara et al., 2018). ChromoPainterV2 software was used to calculate the amount of DNA sequence that is donated from a donor to a recipient group (Lawson et al., 2012). Briefly, for each donor–recipient pair, SNPs in which >90% recipient individuals had recombined with the donor group were considered in the analysis. These SNPs were mapped to genomic regions and specific genes were identified. A total of 258,444 (96.83%) recombining SNPs mapped to 558 genes of the NCTC11168 reference strain with >90% probability of copying from a donor to a recipient strain. Genes containing the highest number of recombining SNPs were considered for subsequent analyses (>95th percentile) (Supplementary file 2). ClonalFrameML (Didelot and Wilson, 2015) was used to infer the relative number of substitutions introduced by recombination (r) and mutation (m) as the ratio r/m as previously described (Mourkas et al., 2020).
Acknowledgements
This work was supported by Wellcome Trust grants 088786/C/09/Z and Medical Research Council (MRC) grants MR/M501608/1 and MR/L015080/1 awarded to SKS. The computational calculations were performed at the Human Genome Center at the Institute of Medical Science (University of Tokyo) and the National Institute of Genetics.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Samuel K Sheppard, Email: s.k.sheppard@bath.ac.uk.
Ben S Cooper, Mahidol University, Thailand.
Gisela Storz, National Institute of Child Health and Human Development, United States.
Funding Information
This paper was supported by the following grants:
Medical Research Council MR/M501608/1 to Samuel K Sheppard.
Medical Research Council MR/L015080/1 to Samuel K Sheppard.
Wellcome Trust 088786/C/09/Z to Samuel K Sheppard.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing - original draft, Writing – review and editing.
Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – review and editing.
Data curation, Formal analysis, Methodology, Software, Writing – review and editing.
Data curation, Formal analysis.
Data curation, Formal analysis.
Data curation, Formal analysis.
Data curation, Formal analysis.
Data curation.
Conceptualization.
Data curation, Resources.
Data curation, Formal analysis.
Data curation, Formal analysis.
Data curation, Resources.
Data curation, Resources.
Resources.
Data curation, Resources.
Conceptualization, Data curation, Formal analysis, Supervision, Validation, Writing – review and editing.
Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review and editing.
Additional files
Data availability
Genomes sequenced as part of other studies are archived on the Short Read Archive associated with BioProject accessions: PRJNA176480, PRJNA177352, PRJNA342755, PRJNA345429, PRJNA312235, PRJNA415188, PRJNA524300, PRJNA528879, PRJNA529798, PRJNA575343, PRJNA524315 and PRJNA689604. Additional genomes were also downloaded from NCBI and pubMLST (http://pubmlst.org/campylobacter). Contiguous assemblies of all genome sequences compared are available at the public data repository Figshare (doi: 10.6084/m9.figshare.15061017) and individual project and accession numbers can be found in Supplementary file 1.
The following dataset was generated:
Pascoe B. 2021. The ecology of interspecies recombination among the zoonotic bacterium Campylobacter. figshare.
References
- Abril C, Brodard I, Perreten V. Two novel antibiotic resistance genes, tet(44) and ant(6)-Ib, are located within a transferable pathogenicity island in Campylobacter fetus subsp fetus. Antimicrobial Agents and Chemotherapy. 2010;54:3052–3055. doi: 10.1128/AAC.00304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Benford D, Cockburn A, Cravedi J, Dogliotti E, Di DA. Nitrite as undesirable substances in animal feed ‐ Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA Journal. 2009;7:1–47. doi: 10.2903/j.efsa.2009.1017. [DOI] [Google Scholar]
- Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, Wishart DS. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Research. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterby C, Mourkas E, Méric G, Pascoe B, Wang H, Waldenström J, Sheppard SK, Olsen B, Järhult JD, Ellström P. The Potential of Isolation Source to Predict Colonization in Avian Hosts: A Case Study in Campylobacter jejuni Strains From Three Bird Species. Frontiers in Microbiology. 2018;9:e9. doi: 10.3389/fmicb.2018.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KS, Dallman TJ, Field N, Childs T, Mitchell H, Day M, Weill FX, Lefèvre S, Tourdjman M, Hughes G, Jenkins C, Thomson N. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nature Communications. 2018;9:1462. doi: 10.1038/s41467-018-03949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. PNAS. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss SC, Thorpe HA, Coyle NM, Sheppard SK, Feil EJ. PIRATE: A fast and scalable pangenomics toolbox for clustering diverged orthologues in bacteria. GigaScience. 2019;8:1–9. doi: 10.1093/gigascience/giz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SL, Maier RJ. Site-directed mutagenesis of Campylobacter concisus respiratory genes provides insight into the pathogen’s growth requirements. Scientific Reports. 2018;8:14203. doi: 10.1038/s41598-018-32509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni MF, Lemey P, Jiang X, Lam TT-Y, Perry BW, Castoe TA, Rambaut A, Robertson DL. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nature Microbiology. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Budroni S, Siena E, Dunning Hotopp JC, Seib KL, Serruto D, Nofroni C, Comanducci M, Riley DR, Daugherty SC, Angiuoli SV, Covacci A, Pizza M, Rappuoli R, Moxon ER, Tettelin H, Medini D. Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. PNAS. 2011;108:4494–4499. doi: 10.1073/pnas.1019751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K-H, Kim K-J. Applications of transposon-based gene delivery system in bacteria. Journal of Microbiology and Biotechnology. 2009;19:217–228. doi: 10.4014/jmb.0811.669. [DOI] [PubMed] [Google Scholar]
- Cody AJ, McCarthy ND, Bray JE, Wimalarathna HML, Colles FM, Jansen van Rensburg MJ, Dingle KE, Waldenström J, Maiden MCJ. Wild bird-associated Campylobacter jejuni isolates are a consistent source of human disease, in Oxfordshire, United Kingdom. Environmental Microbiology Reports. 2015;7:782–788. doi: 10.1111/1758-2229.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles FM, Dingle KE, Cody AJ, Maiden MCJ. Comparison of Campylobacter populations in wild geese with those in starlings and free-range poultry on the same farm. Applied and Environmental Microbiology. 2008;74:3583–3590. doi: 10.1128/AEM.02491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles FM, McCarthy ND, Bliss CM, Layton R, Maiden MCJ. The long-term dynamics of Campylobacter colonizing a free-range broiler breeder flock: an observational study. Environmental Microbiology. 2015;17:938–946. doi: 10.1111/1462-2920.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLOS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearlove BL, Cody AJ, Pascoe B, Méric G, Wilson DJ, Sheppard SK. Rapid host switching in generalist Campylobacter strains erodes the signal for tracing human infections. The ISME Journal. 2016;10:721–729. doi: 10.1038/ismej.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne L, On SLW, De Brandt E, Vandamme P. Novel Campylobacter lari-like bacteria from humans and molluscs: description of Campylobacter peloridis sp. International Journal of Systematic and Evolutionary Microbiology. 2009;59:1126–1132. doi: 10.1099/ijs.0.000851-0. [DOI] [PubMed] [Google Scholar]
- Didelot X, Achtman M, Parkhill J, Thomson NR, Falush D. A bimodal pattern of relatedness between the Salmonella Paratyphi A and Typhi genomes: convergence or divergence by homologous recombination? Genome Research. 2007;17:61–68. doi: 10.1101/gr.5512906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X, Wilson DJ. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLOS Computational Biology. 2015;11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle KE, Colles FM, Wareing DRA, Ure R, Fox AJ, Bolton FE, Bootsma HJ, Willems RJL, Urwin R, Maiden MCJ. Multilocus Sequence Typing System for Campylobacter jejuni. Journal of Clinical Microbiology. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle WF, Papke RT. Genomics and the bacterial species problem. Genome Biology. 2006;7:116. doi: 10.1186/gb-2006-7-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle WF, Zhaxybayeva O. On the origin of prokaryotic species. Genome Research. 2009;19:744–756. doi: 10.1101/gr.086645.108. [DOI] [PubMed] [Google Scholar]
- Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, Sorek R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science (New York, N.Y.) 2018;359:eaar4120. doi: 10.1126/science.aar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston AK, Mitchell AH, West SC. In vitro reconstitution of the late steps of genetic recombination in E. coli. Cell. 1997;89:607–617. doi: 10.1016/s0092-8674(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald C, Tu ZC, Patrick M, Stiles T, Lawson AJ, Santovenia M, Gilbert MJ, van Bergen M, Joyce K, Pruckler J, Stroika S, Duim B, Miller WG, Loparev V, Sinnige JC, Fields PI, Tauxe RV, Blaser MJ, Wagenaar JA. Campylobacter fetus subsp testudinum subsp nov., isolated from humans and reptiles. International Journal of Systematic and Evolutionary Microbiology. 2014;64:2944–2948. doi: 10.1099/ijs.0.057778-0. [DOI] [PubMed] [Google Scholar]
- Fraser C, Hanage WP, Spratt BG. Recombination and the nature of bacterial speciation. Science (New York, N.Y.) 2007;315:476–480. doi: 10.1126/science.1127573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli M, Piccirillo A. Pet reptiles as potential reservoir of Campylobacter species with zoonotic potential. The Veterinary Record. 2014;174:479. doi: 10.1136/vr.102243. [DOI] [PubMed] [Google Scholar]
- Gilbert MJ, Kik M, Miller WG, Duim B, Wagenaar JA. Campylobacter iguaniorum sp. nov., isolated from reptiles. International Journal of Systematic and Evolutionary Microbiology. 2015;65:975–982. doi: 10.1099/ijs.0.000048. [DOI] [PubMed] [Google Scholar]
- Gilbert MJ, Miller WG, Leger JS, Chapman MH, Timmerman AJ, Duim B. Campylobacter pinnipediorum sp nov, isolated from pinnipeds, comprising Campylobacter pinnipediorum subsp pinnipediorum subsp. Nov. and Campylobacter Pinnipediorum Subsp. Caledonicus Subsp. Nov. Int J Syst Evol Microbiol. 2017;67:1961–1968. doi: 10.1099/ijsem.0.001894. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. Hybridization of bird species. Science (New York, N.Y.) 1992;256:193–197. doi: 10.1126/science.256.5054.193. [DOI] [PubMed] [Google Scholar]
- Griekspoor P, Colles FM, McCarthy ND, Hansbro PM, Ashhurst-Smith C, Olsen B, Hasselquist D, Maiden MCJ, Waldenström J. Marked host specificity and lack of phylogeographic population structure of Campylobacter jejuni in wild birds. Molecular Ecology. 2013;22:1463–1472. doi: 10.1111/mec.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. Journal of Medical Microbiology. 2005;54:1093–1101. doi: 10.1099/jmm.0.45935-0. [DOI] [PubMed] [Google Scholar]
- Howlett RM, Hughes BM, Hitchcock A, Kelly DJ. Hydrogenase activity in the foodborne pathogen Campylobacter jejuni depends upon a novel ABC-type nickel transporter (NikZYXWV) and is SlyD-independent. Microbiology (Reading, England) 2012;158:1645–1655. doi: 10.1099/mic.0.054130-0. [DOI] [PubMed] [Google Scholar]
- Iraola G, Forster SC, Kumar N, Lehours P, Bekal S, García-Peña FJ, Paolicchi F, Morsella C, Hotzel H, Hsueh P-R, Vidal A, Lévesque S, Yamazaki W, Balzan C, Vargas A, Piccirillo A, Chaban B, Hill JE, Betancor L, Collado L, Truyers I, Midwinter AC, Dagi HT, Mégraud F, Calleros L, Pérez R, Naya H, Lawley TD. Distinct Campylobacter fetus lineages adapted as livestock pathogens and human pathobionts in the intestinal microbiota. Nature Communications. 2017;8:1367. doi: 10.1038/s41467-017-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nature Communications. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FSL, Wright GD, McArthur AG. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Research. 2017;45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. Journal of Medical Microbiology. 2013;62:499–513. doi: 10.1099/jmm.0.052555-0. [DOI] [PubMed] [Google Scholar]
- Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clinical Microbiology Reviews. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KF, Méric G, Nielsen HL, Pascoe B, Sheppard SK, Thorlacius-Ussing O, Nielsen H. Molecular epidemiology and comparative genomics of Campylobacter concisus strains from saliva, faeces and gut mucosal biopsies in inflammatory bowel disease. Scientific Reports. 2018;8:e4. doi: 10.1038/s41598-018-20135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DJ, Hellenthal G, Myers S, Falush D. Inference of population structure using dense haplotype data. PLOS Genetics. 2012;8:e1002453. doi: 10.1371/journal.pgen.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherbarrow AJH, Griffiths R, Hart CA, Kemp R, Williams NJ, Diggle PJ, Wright EJ, Sutherst J, Houghton P, French NP. Campylobacter lari: genotype and antibiotic resistance of isolates from cattle, wildlife and water in an area of mixed dairy farmland in the United Kingdom. Environmental Microbiology. 2007;9:1772–1779. doi: 10.1111/j.1462-2920.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Li X, Xie Y, Bi D, Sun J, Li J, Tai C, Deng Z, Ou H-Y. ICEberg 2.0: an updated database of bacterial integrative and conjugative elements. Nucleic Acids Research. 2019;47:D660–D665. doi: 10.1093/nar/gky1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM. Introduction: the challenge of multiresistance. International Journal of Antimicrobial Agents. 2007;29 Suppl 3:S1–S7. doi: 10.1016/S0924-8579(07)00158-6. [DOI] [PubMed] [Google Scholar]
- Logan JMJ, Burnens A, Linton D, Lawson AJ, Stanley J. Campylobacter lanienae sp. nov., a new species isolated from workers in an abattoir. International Journal of Systematic and Evolutionary Microbiology. 2000;50 Pt 2:865–872. doi: 10.1099/00207713-50-2-865. [DOI] [PubMed] [Google Scholar]
- Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Applied and Environmental Microbiology. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Sahin O, Lin J, Michel LO, Zhang Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrobial Agents and Chemotherapy. 2003;47:390–394. doi: 10.1128/AAC.47.1.390-394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- Man SM. The clinical importance of emerging Campylobacter species. Nature Reviews. Gastroenterology & Hepatology. 2011;8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- Margus T, Remm M, Tenson T. Phylogenetic distribution of translational GTPases in bacteria. BMC Genomics. 2007;8:1–18. doi: 10.1186/1471-2164-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science (New York, N.Y.) 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney JO, Whelan FJ, Domingo-Sananes MR, McNally A, O’Connell MJ. In: The Pangenome. Tettelin H, Medini D, editors. Springer; 2020. Pangenomes and Selection: The Public Goods Hypothesis; pp. 151–167. [DOI] [PubMed] [Google Scholar]
- McLennan MK, Ringoir DD, Frirdich E, Svensson SL, Wells DH, Jarrell H, Szymanski CM, Gaynor EC. Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide. Journal of Bacteriology. 2008;190:1097–1107. doi: 10.1128/JB.00516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheil DM, Shippy DC, Eakley NM, Okwumabua OE, Fadl AA. Deletion of gene encoding methyltransferase (gidB) confers high-level antimicrobial resistance in Salmonella. The Journal of Antibiotics. 2012;65:185–192. doi: 10.1038/ja.2012.5. [DOI] [PubMed] [Google Scholar]
- Miller WG, Yee E, Chapman MH, Smith TPL, Bono JL, Huynh S, Parker CT, Vandamme P, Luong K, Korlach J. Comparative genomics of the Campylobacter lari group. Genome Biology and Evolution. 2014;6:3252–3266. doi: 10.1093/gbe/evu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkas E, Florez-Cuadrado D, Pascoe B, Calland JK, Bayliss SC, Mageiros L, Méric G, Hitchings MD, Quesada A, Porrero C, Ugarte-Ruiz M, Gutiérrez-Fernández J, Domínguez L, Sheppard SK. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environmental Microbiology. 2019;21:4597–4613. doi: 10.1111/1462-2920.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkas E, Taylor AJ, Méric G, Bayliss SC, Pascoe B, Mageiros L, Calland JK, Hitchings MD, Ridley A, Vidal A, Forbes KJ, Strachan NJC, Parker CT, Parkhill J, Jolley KA, Cody AJ, Maiden MCJ, Kelly DJ, Sheppard SK. Agricultural intensification and the evolution of host specialism in the enteric pathogen Campylobacter jejuni. PNAS. 2020;117:11018–11028. doi: 10.1073/pnas.1917168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi T, Holden MTG, Holden MTG, Didelot X, Mehershahi K, Boddey JA, Beacham I, Peak I, Harting J, Baybayan P, Guo Y, Wang S, How LC, Sim B, Essex-Lopresti A, Sarkar-Tyson M, Nelson M, Smither S, Ong C, Aw LT, Hoon CH, Michell S, Studholme DJ, Titball R, Chen SL, Parkhill J, Tan P. Burkholderia pseudomallei sequencing identifies genomic clades with distinct recombination, accessory, and epigenetic profiles. Genome Research. 2015;25:129–141. doi: 10.1101/gr.177543.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. The Journal of Infectious Diseases. 2005;191:1078–1083. doi: 10.1086/427996. [DOI] [PubMed] [Google Scholar]
- Oliveira PH, Touchon M, Rocha EPC. Regulation of genetic flux between bacteria by restriction-modification systems. PNAS. 2016;113:5658–5663. doi: 10.1073/pnas.1603257113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, Harris SR. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microbial Genomics. 2016;2:e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Research. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe B, Méric G, Yahara K, Wimalarathna H, Murray S, Hitchings MD, Sproston EL, Carrillo CD, Taboada EN, Cooper KK, Huynh S, Cody AJ, Jolley KA, Maiden MCJ, McCarthy ND, Didelot X, Parker CT, Sheppard SK. Local genes for local bacteria: Evidence of allopatry in the genomes of transatlantic Campylobacter populations. Molecular Ecology. 2017;26:4497–4508. doi: 10.1111/mec.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe B, Williams LK, Calland JK, Meric G, Hitchings MD, Dyer M, Ryder J, Shaw S, Lopes BS, Chintoan-Uta C, Allan E, Vidal A, Fearnley C, Everest P, Pachebat JA, Cogan TA, Stevens MP, Humphrey TJ, Wilkinson TS, Cody AJ, Colles FM, Jolley KA, Maiden MCJ, Strachan N, Pearson BM, Linton D, Wren BW, Parkhill J, Kelly DJ, van Vliet AHM, Forbes KJ, Sheppard SK. Domestication of Campylobacter jejuni NCTC 11168. Microbial Genomics. 2019;5:e5. doi: 10.1099/mgen.0.000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo A, Niero G, Calleros L, Pérez R, Naya H, Iraola G. Campylobacter geochelonis sp nov isolated from the western Hermann’s tortoise (Testudo hermanni hermanni) International Journal of Systematic and Evolutionary Microbiology. 2016;66:3468–3476. doi: 10.1099/ijsem.0.001219. [DOI] [PubMed] [Google Scholar]
- Pittman MS, Elvers KT, Lee L, Jones MA, Poole RK, Park SF, Kelly DJ. Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Molecular Microbiology. 2007;63:575–590. doi: 10.1111/j.1365-2958.2006.05532.x. [DOI] [PubMed] [Google Scholar]
- Price LB, Koch BJ, Hungate BA. Ominous projections for global antibiotic use in food-animal production. PNAS. 2015;112:5554–5555. doi: 10.1073/pnas.1505312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J, Nash JHE. MOB-suite: software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microbial Genomics. 2018;4:e4. doi: 10.1099/mgen.0.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J, Bessonov K, Schonfeld J, Nash JHE. Universal whole-sequence-based plasmid typing and its utility to prediction of host range and epidemiological surveillance. Microbial Genomics. 2020;6:1–12. doi: 10.1099/mgen.0.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl JW, Caporaso JG, Rasko DA, Keim P. The large-scale blast score ratio (LS-BSR) pipeline: a method to rapidly compare genetic content between bacterial genomes. PeerJ. 2014;2:e332. doi: 10.7717/peerj.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat: Table 1. Journal of Antimicrobial Chemotherapy. 2016;71:2066–2070. doi: 10.1093/jac/dkw274. [DOI] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics (Oxford, England) 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Semanjski M, Germain E, Bratl K, Kiessling A, Gerdes K, Macek B. The kinases HipA and HipA7 phosphorylate different substrate pools in Escherichia coli to promote multidrug tolerance. Science Signaling. 2018;11:e11. doi: 10.1126/scisignal.aat5750. [DOI] [PubMed] [Google Scholar]
- Shapiro BJ, Leducq JB, Mallet J. What Is Speciation? PLOS Genetics. 2016;12:e1005860. doi: 10.1371/journal.pgen.1005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, McCarthy ND, Falush D, Maiden MCJ. Convergence of Campylobacter species: implications for bacterial evolution. Science (New York, N.Y.) 2008;320:237–239. doi: 10.1126/science.1155532. [DOI] [PubMed] [Google Scholar]
- Sheppard SK, Dallas JF, MacRae M, McCarthy ND, Sproston EL, Gormley FJ, Strachan NJC, Ogden ID, Maiden MCJ, Forbes KJ. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. International Journal of Food Microbiology. 2009a;134:96–103. doi: 10.1016/j.ijfoodmicro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Dallas JF, Strachan NJC, MacRae M, McCarthy ND, Wilson DJ, Gormley FJ, Falush D, Ogden ID, Maiden MCJ, Forbes KJ. Campylobacter genotyping to determine the source of human infection. Clinical Infectious Diseases. 2009b;48:1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Colles F, Richardson J, Cody AJ, Elson R, Lawson A, Brick G, Meldrum R, Little CL, Owen RJ, Maiden MCJ, McCarthy ND. Host association of Campylobacter genotypes transcends geographic variation. Applied and Environmental Microbiology. 2010;76:5269–5277. doi: 10.1128/AEM.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Colles FM, McCarthy ND, Strachan NJC, Ogden ID, Forbes KJ, Dallas JF, Maiden MCJ. Niche segregation and genetic structure of Campylobacter jejuni populations from wild and agricultural host species. Molecular Ecology. 2011a;20:3484–3490. doi: 10.1111/j.1365-294X.2011.05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, McCarthy ND, Jolley KA, Maiden MCJ. Introgression in the genus Campylobacter: generation and spread of mosaic alleles. Microbiology (Reading, England) 2011b;157:1066–1074. doi: 10.1099/mic.0.045153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Jolley KA, Maiden MCJ. A Gene-By-Gene Approach to Bacterial Population Genomics: Whole Genome MLST of Campylobacter. Genes. 2012;3:261–277. doi: 10.3390/genes3020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Didelot X, Jolley KA, Darling AE, Pascoe B, Meric G, Kelly DJ, Cody A, Colles FM, Strachan NJC, Ogden ID, Forbes K, French NP, Carter P, Miller WG, McCarthy ND, Owen R, Litrup E, Egholm M, Affourtit JP, Bentley SD, Parkhill J, Maiden MCJ, Falush D. Progressive genome-wide introgression in agricultural Campylobacter coli. Molecular Ecology. 2013;22:1051–1064. doi: 10.1111/mec.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Cheng L, Méric G, de Haan CPA, Llarena A-K, Marttinen P, Vidal A, Ridley A, Clifton-Hadley F, Connor TR, Strachan NJC, Forbes K, Colles FM, Jolley KA, Bentley SD, Maiden MCJ, Hänninen M-L, Parkhill J, Hanage WP, Corander J. Cryptic ecology among host generalist Campylobacter jejuni in domestic animals. Molecular Ecology. 2014;23:2442–2451. doi: 10.1111/mec.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Guttman DS, Fitzgerald DS. Population genomics of bacterial host adaptation. Nature Reviews. Genetics. 2018;19:549–565. doi: 10.1038/s41576-018-0032-z. [DOI] [PubMed] [Google Scholar]
- Smith JM, Dowson CG, Spratt BG. Localized sex in bacteria. Nature. 1991;349:29–31. doi: 10.1038/349029a0. [DOI] [PubMed] [Google Scholar]
- Sproston EL, Wimalarathna HML, Sheppard SK. Trends in fluoroquinolone resistance in Campylobacter. Microbial Genomics. 2018;4:1–8. doi: 10.1099/mgen.0.000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, Macpherson AJ, Hardt W-D. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLOS Pathogens. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski CM, Logan SM, Linton D, Wren BW. Campylobacter--a tale of two protein glycosylation systems. Trends in Microbiology. 2003;11:233–238. doi: 10.1016/s0966-842x(03)00079-9. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Méric G, Yahara K, Pascoe B, Mageiros L, Mourkas E, Calland JK, Puranen S, Hitchings MD, Bayliss S, Jolley KA, Kobras CM, Williams NJ, van Vliet AHM, Parkhill J, Maiden MCJ, Corander J, Falush D, Didelot X, Kelly DJ, Sheppard SK. Cross-Species Evolutionary Rewiring in the Enteric Bacterium Campylobacter. bioRxiv. 2021 doi: 10.1101/2021.03.15.435406. [DOI]
- Teuber M. Veterinary use and antibiotic resistance. Current Opinion in Microbiology. 2001;4:493–499. doi: 10.1016/s1369-5274(00)00241-1. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nature Reviews. Microbiology. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Thorell K, Yahara K, Berthenet E, Lawson DJ, Mikhail J, Kato I, Mendez A, Rizzato C, Bravo MM, Suzuki R, Yamaoka Y, Torres J, Sheppard SK, Falush D. Rapid evolution of distinct Helicobacter pylori subpopulations in the Americas. PLOS Genetics. 2017;13:e1006546. doi: 10.1371/journal.pgen.1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van TTH, Elshagmani E, Gor MC, Scott PC, Moore RJ. Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. International Journal of Systematic and Evolutionary Microbiology. 2016;66:4518–4524. doi: 10.1099/ijsem.0.001383. [DOI] [PubMed] [Google Scholar]
- van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. PNAS. 2012;109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M, Eyre-Walker A. Are pangenomes adaptive or not? Nature Microbiology. 2017;2:1576. doi: 10.1038/s41564-017-0067-5. [DOI] [PubMed] [Google Scholar]
- Werren JH. Selfish genetic elements, genetic conflict, and evolutionary innovation. PNAS. 2011;108 Suppl 2:10863–10870. doi: 10.1073/pnas.1102343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock DJ, Krusche P, Strachan NJC, Forbes KJ, Cohan FM, Méric G, Sheppard SK. Genomic plasticity and rapid host switching can promote the evolution of generalism: a case study in the zoonotic pathogen Campylobacter. Scientific Reports. 2017;7:9650. doi: 10.1038/s41598-017-09483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara K, Furuta Y, Oshima K, Yoshida M, Azuma T, Hattori M, Uchiyama I, Kobayashi I. Chromosome painting in silico in a bacterial species reveals fine population structure. Molecular Biology and Evolution. 2013;30:1454–1464. doi: 10.1093/molbev/mst055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara K, Nakayama S-I, Shimuta K, Lee K-I, Morita M, Kawahata T, Kuroki T, Watanabe Y, Ohya H, Yasuda M, Deguchi T, Didelot X, Ohnishi M. Genomic surveillance of Neisseria gonorrhoeae to investigate the distribution and evolution of antimicrobial-resistance determinants and lineages. Microbial Genomics. 2018;4:1–3. doi: 10.1099/mgen.0.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su CC, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J. Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics. MBio. 2016;7:e16. doi: 10.1128/mBio.01543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JPW. Bacteria Are Smartphones and Mobile Genes Are Apps. Trends in Microbiology. 2016;24:931–932. doi: 10.1016/j.tim.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. Identification of acquired antimicrobial resistance genes. The Journal of Antimicrobial Chemotherapy. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, van der Ende A, Falush D, Brieske N, Morelli G, Linz B, Popovic T, Schuurman IG, Adegbola RA, Zurth K, Gagneux S, Platonov AE, Riou JY, Caugant DA, Nicolas P, Achtman M. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. PNAS. 2001;98:5234–5239. doi: 10.1073/pnas.061386098. [DOI] [PMC free article] [PubMed] [Google Scholar]