Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of l‐glutamic acid and monosodium l‐glutamate monohydrate produced by fermentation using the genetically modified strain Corynebacterium glutamicum NITE BP‐01681. The additives are intended to be used in feed and water for drinking for all animal species and categories as nutritional additives (amino acids) or as sensory additives (flavouring compounds). Viable cells of the production strain and its DNA were not detected in the final additives. The additives do not give rise to any safety concern regarding the production strain. l‐Glutamic acid and monosodium l‐glutamate monohydrate produced using C. glutamicum NITE BP‐01681 are considered safe for the target species, for the consumer and for the environment. However, the Panel raised concerns on the use in water for drinking for hygienic reasons. The additives are considered not irritant to skin or eyes and not dermal sensitisers but a risk by inhalation. The Panel concluded that the additives are efficacious as nutritional additives and as flavouring compounds.
Keywords: l‐glutamic acid, monosodium l‐glutamate monohydrate, ‘amino acids their salts and analogues’, flavouring compounds, Corynebacterium glutamicum, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Ajinomoto Animal Nutrition Europe 2 for authorisation of the product l‐glutamic acid and its monosodium salt produced by Corynebacterium glutamicum NITE BP‐01681, when used as a feed additive for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues; and category: sensory additives; functional group: flavouring compounds).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 13 November 2020.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐glutamic acid and its sodium salt produced by Corynebacterium glutamicum NITE BP‐01681, when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
l‐Glutamic acid and monosodium l‐glutamate monohydrate (minimum 98% dry matter (DM)) produced by fermentation using a genetically modified strain of C. glutamicum, NITE BP‐01681, have not been authorised in the European Union.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 3 in support of the authorisation request for the use of l‐glutamic acid and monosodium l‐glutamate produced by Corynebacterium glutamicum NITE BP‐01681 as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the L‐glutamic acid and monosodium glutamate in animal feed. The Executive Summary of the EURL report can be found in Annex A. 4
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐glutamic acid and monosodium l‐glutamate is in line with the principles laid down in Regulation (EC) No 429/2008 5 and the relevant guidance documents: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018b) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
l‐Glutamic acid and monosodium l‐glutamate monohydrate produced by Corynebacterium glutamicum NITE BP‐01681 are intended to be used as sensory additives (functional group: flavouring compounds) or as nutritional additives (functional group: amino acids, their salts and analogues) in feed and water for drinking for all animal species.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The l‐glutamic acid present or used in the additives under assessment is produced by a genetically modified strain of Corynebacterium glutamicum, which is deposited in the National Institute of Technology and Evaluation (NITE) of Japan with accession number NITE BP‐01681. 6
The genome of the production strain NITE BP‐01681 and that of the ■■■■■ parental strain C. glutamicum ■■■■■ present in the Company’s Group collection were compared to a publicly available genome of the parental strain that was used as reference (■■■■■). 7 The taxonomic identification of the production strain as C. glutamicum was confirmed by average nucleotide identity (ANI) analysis of the whole genome sequence (WGS) data of the production strain with an ANI value of 99.97% and 99.99% compared to the publicly available genome of the parental strain and the genome sequence of the company´s cultivar, respectively.
The production strain and its ■■■■■ were tested for their susceptibility to the antimicrobials listed for ‘Corynebacterium and other Gram‐positive’ in the Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a). 8 All minimum inhibitory concentration values were below or equal to the reference values and no differences were identified between the parental and the production strain. Therefore, the production strain is considered susceptible to all relevant antimicrobials.
The WGS data of the production and parental strains were searched for the presence of antimicrobial resistance (AMR) genes and genes encoding for virulence determinants. 9 ■■■■■ No hits of concern were identified.
The presence of genes encoding for virulence determinants was checked ■■■■■ 9 ■■■■■ No hits of concern were identified.
3.1.1.1. Information related to the genetically modified microorganism
Characterisation of the parental or recipient microorganism
The parental strain is ■■■■■ 10
Characterisation of the donor organisms
■■■■■
Description of the genetic modification
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
The additives l‐glutamic acid and monosodium l‐glutamate monohydrate are produced by fed‐batch fermentation with Corynebacterium glutamicum NITE BP‐01681. ■■■■■ The monosodium l‐glutamate monohydrate can be presented in different forms depending on the sieving applied: selection, fine and powder. The applicant states that no antimicrobial substances are used in the manufacturing of the additives.
3.1.3. Characterisation of the additives
3.1.3.1. l‐Glutamic acid
l‐Glutamic acid (International Union of Pure and Applied Chemistry (IUPAC) name: 2‐aminopentanedioic acid) is identified with the Chemical Abstracts Service (CAS) No 56‐86‐0 and the European Inventory of Existing Commercial Chemical Substances (EINECS) No 200‐293‐7, and has a molecular mass of 147.13 g/mol. The molecular formula of l‐glutamic acid is C5H9O4N and the structural formula is given in Figure 1.
Figure 1.

Structural formula of l‐glutamic acid
The additive contains by specification not less than 98% l‐glutamic acid, expressed on dry matter basis. The analysis of eight batches ■■■■■, which supports the specifications set by the applicant. 12
The specific optical rotation at 20°C was measured in five batches and showed an average value of +31.9°, 13 which is within the range for l‐glutamic acid (+31.5° to +32.2°, pubChem), 14 confirming the identity of the l‐enantiomer in accordance with the specifications set for l‐glutamic acid (E620) as a food additive. 15
Three batches of the additive were analysed for heavy metals (cadmium, lead and mercury), arsenic, fluorine, melamine, hydrocyanic acid, aflatoxins (B1, B2, G1 and G2), zearalenone, deoxynivalenol, ochratoxin A, T‐2 toxin, HT‐2 toxin and fumonisins (B1, B2 and B3). All analysed values for the above were below the respective LOQ. 18 Concentrations of pesticides, namely organochlorine (including pyrethroids) and organophosphorus, were analysed in three batches and results were all lower than the LOQ. 19 The sum of polychlorinated dibenzodioxins/dibenzofurans (PCDD/F) and dioxin‐like polychlorinated biphenyls (PCB) was analysed in three batches and ranged from 0.0833 to 0.0881 ng WHO 2005 TEQ/kg. The sum of dioxin‐like PCBs, except PCB 118 (three batches analysed), ranged from 0.0499 to 0.0524 WHO 2005 TEQ µg/kg additive. 20
Five batches were tested for microbial contamination 21 and the results showed total bacterial count and presumptive Bacillus cereus (at 30°C) were < 100 colony forming unit (CFU)/g, Salmonella spp. were not detected in 25 g, while coagulase‐positive staphylococci (including Staphylococcus aureus), coliforms (at 30°C), Enterobacteriaceae (at 37°C), yeasts and filamentous fungi (at 25°C) were < 10 CFU/g.
The inhibitory activity of l‐glutamic acid on microorganisms was tested (three batches using the broth dilution method) against the following reference strains: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212 and Bacillus subtilis ATCC 6633. 22 No inhibition was observed after 48 h incubation, denoting the lack of antimicrobial activity.
The presence of viable cells of the production strain was investigated in three batches of l‐glutamic acid, each batch tested in triplicate. 23 ■■■■■ No growth of the production strain was detected in the samples tested.
The presence of DNA of the production strain was tested in three batches of the final l‐glutamic acid product, each tested in triplicate. 24 ■■■■■ No DNA was detected in the batches analysed.
The additive appears as crystals or crystalline white powder with a solubility in water at 25°C of 8.64 g/L. The dusting potential of the additive measured in three batches following the Stauber‐Heubach method and analytical values ranged from 758 to 928 mg/m3 ■■■■■ 25 ■■■■■ 26
3.1.3.2. Monosodium l‐glutamate monohydrate
Monosodium l‐glutamate monohydrate (IUPAC name: sodium 2‐aminopentanedioate monohydrate (synonyms: glutamic acid monosodium salt monohydrate, l‐glutamic acid sodium salt hydrate (1:1:1))) is identified with the CAS No 6106‐04‐3 and the EINECS No 205‐538‐1, and has a molecular mass of 187.13 g/mol. The molecular formula of monosodium l‐glutamate is C5H8NNaO4·H2O and the structural formula is given in Figure 2.
Figure 2.
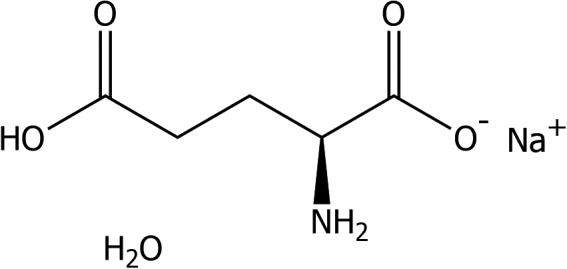
Structural formula of monosodium glutamate monohydrate
The additive contains by specification not less than 98% monosodium glutamate monohydrate expressed on dry matter (with Na representing 12.29%, crystallisation/hydration water 9.63% and glutamic acid monoanion 78.08%). Three different forms were described: selection, fine or powder. The data presented here below are for the selection form, unless otherwise stated and may be representative for the three different forms of the additive.
■■■■■ 27 ■■■■■ 28 The analysis of different batches supports the specification set by the applicant.
The specific optical rotation was measured in three batches of the final product and the average was +25.2° (range +25.2° to +25.3°), 29 which is within the range for monosodium glutamate monohydrate (+24.8° to +25.3°, PubChem), 30 demonstrates the identity of the l‐enantiomer and it is in accordance with the specifications set for monosodium glutamate (E621) as a food additive.15
Three batches of the additive were analysed for chemical contamination which included different substances. 33 Heavy metals (cadmium, lead and mercury), arsenic, fluorine, melamine, hydrocyanic acid, aflatoxins (B1, B2, G1 and G2), zearalenone, deoxynivalenol, ochratoxin A, T‐2 toxin, HT‐2 toxin and fumonisins (B1, B2 and B3) were below the respective LOQ. 34 The samples were also analysed for pesticides, namely organochlorine (including pyrethroids) and organophosphates were analysed in three batches and resulted all below LOQ. 35 In these three batches, the sum of PCDD/F and dioxin‐like PCBs was analysed in three batches and ranged from 0.0667 to 0.0783 ng WHO 2005 TEQ /kg. The sum of dioxin‐like PCBs, except PCB 118 (three batches analysed) ranged from 0.0396 to 0.0458 WHO 2005 TEQ µg/kg additive.
Five batches were analysed for microbial contamination 36 and showed total bacterial count and presumptive Bacillus cereus (at 30°C) < 100 colony forming unit (CFU)/g, Salmonella spp. not detected in 25 g, while coagulase‐positive staphylococci (including Staphylococcus aureus), coliforms (at 30°C), Enterobacteriaceae (at 37°C), yeasts and filamentous fungi (at 25°C) were < 10 CFU/g.
The inhibitory activity on microorganisms was tested in three batches of monosodium glutamate monohydrate with the broth dilution method against the following reference strains: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212 and Bacillus subtilis ATCC 6633. 37 No inhibition was observed after 48 h incubation, denoting the lack of antimicrobial activity.
The presence of viable cells of the production strain was tested in three batches of the additive, each batch tested in triplicate. 38 ■■■■■ No growth of the production strain was found in the samples tested.
The presence of DNA of the production strain was tested in three batches of the monosodium glutamate, each tested in triplicated. 39 ■■■■■ No DNA was detected in the batches analysed.
The additive appears as crystals or fine crystalline white powder with a solubility in water of 417 g/L. The dusting potential of the additive measured in three batches of the selection form following the Stauber‐Heubach method ranged from 278 to 345 mg/m3 ■■■■■
3.1.4. Stability and homogeneity
The stability of l‐glutamic acid was tested in three batches, stored in sealed nylon‐polyethylene bags for 12 months at 25°C and 60% relative humidity (RH) or at 40°C/60% RH. 42 Results showed that l‐glutamic acid concentrations in dry matter basis varied between 99.1% and 100.8%, indicating negligible loss of the content of l‐glutamic acid.
Three batches of the monosodium l‐glutamate (selection grade) were stored for 9 months in sealed bags at 40°C and showed no losses in the content of the active substance. 43 Data for two batches stored for 5 years (storage conditions not indicated) showed no losses in the content of the active substance. 44 Moreover, the applicant also provided information on the shelf‐life for batches of the product produced by fermentation with a different production strain. 45 The purity of the batches was similar to the one reported for the product under assessment. The samples were mainly kept at room temperature, and the monitoring was done for different time periods and up to 10 years. The results showed negligible losses on the content of the active substance and could support the shelf‐life of the additive under assessment.
The stability of l‐glutamic acid in premixtures was assessed in samples stored for 6 months at 25°C/30% RH and at 40°C/60% RH. The three batches were tested (a different batch for each premixture) in three vitamin–mineral premixtures (one for piglets, a second for gestating sows and a third for chickens for fattening) which contained choline chloride (0.8, 1.6 and 2%, respectively). The stability of l‐glutamic acid was studied at inclusion rates of 5%, except for the chickens for fattening premixture that was supplemented with 7%. 46 The maximum losses after 180 days in the premixture for piglets’ feed were 1.9% at 25°C/30% RH and 4.6% at 40°C/60% RH; in the premixture for gestating sows’ were 1.8% at 25°C/30% RH and 1% at 40°C/60% RH; and in the premixture for chickens for fattening were 1.6% at 25°C/30% RH and 1.9% at 40°C/60% RH.
The stability in feed was assessed in three batches of l‐glutamic acid that were added to feed for piglets, for gestating sows and for chickens for fattening, respectively. 47 The L‐glutamic acid was supplemented at 0.375%, 0.30% and 0.225%, respectively. The compound feeds were preconditioned at 59–65°C and subsequently pelleted at 89.4°C, 76°C or 73°C, respectively. After cooling, the feeds were packed in sealed polyethylene nylon bags and stored at 25°C/60% RH and at 40°C/60% RH for 3 months. Pelleting caused a loss of 0.001–0.611%, depending on the feed considered. Losses observed in pelleted feed stored at 25°C/60% RH were 3.6%, 2.4% and 3.8% for feed for piglets, gestating sows and chickens for fattening, respectively. Those observed in pelleted feed stored at 40°C/60% RH were 11.8%, 3.4% and a gain of 28% (possibly due to conversion of l‐glutamine), respectively.
The premixtures46 and feed47 described above were used to study the capacity of the additive to distribute homogeneously. l‐Glutamic acid was analysed in 10 subsamples of each premixture and feed. Total glutamic acid was analysed. The coefficients of variation (CV) of the premixtures were 3.2%, 2.2% and 1.4% for piglet, gestating sows and chickens for fattening, respectively. The CVs of the pelleted feeds were 3.8%, 4.2% and 2.9%, respectively.
The stability of L‐glutamic acid in water was tested with the concentrations of 0.5, 2.5 and 5 g/L (one batch per concentration) at 25°C for 50 h. 48 The amino acid was dissolved in tap water and stirred for 15 min and incubated under the above‐mentioned conditions. The recoveries of l‐glutamic acid were on average 100.3%, 98.9% and 97.4% for the solutions of 0.5, 2.5 and 5 g l‐glutamic acid/L, respectively. The coefficients of variation (CV) were 1.14%, 0.53% and 1.04% for the solutions of 0.5, 2.5 and 5 g l‐glutamic acid/L, respectively.
No data on the stability and capacity to homogeneously distribute of monosodium l‐glutamate in premixtures nor feed/water was provided. 49 However, the data on l‐glutamic acid would be indicative of the stability and capacity to homogeneously distribute for monosodium l‐glutamate monohydrate.
3.1.5. Conditions of use
The additives l‐glutamic acid and monosodium l‐glutamate monohydrate are intended to be used in feed or water for drinking in all animal species as nutritional (amino acids) or as sensory additives (flavouring compounds). The applicant proposed no minimum or maximum content of l‐glutamic acid and monosodium l‐glutamate monohydrate in feed or water for drinking for all animal species. Notwithstanding, the applicant notes that an inclusion level of up to 10 g of l‐glutamic acid or monosodium l‐glutamate monohydrate/kg feed may be reached when used as a sensory additive.
3.2. Safety
3.2.1. Safety of the production organism
The production strain, NITE BP‐01681, belongs to the species C. glutamicum that qualifies for the qualified presumption of safety (QPS) approach to safety assessment (EFSA, 2007) when used for production purposes (EFSA BIOHAZ Panel, 2020). The production strain was unambiguously identified as C. glutamicum. Moreover, it was shown to be susceptible to the relevant antibiotics, not to contain acquired antimicrobial resistance genes, and the genetic modification was considered of no concern. Finally, no viable cells or recombinant DNA of the production strain were detected in the final products. Therefore, the qualifications for QPS approach were met and, consequently, the use of C. glutamicum NITE BP‐01681 is considered safe for the production of l‐glutamic acid and monosodium l‐glutamate monohydrate.
3.2.2. Safety for the target species, consumers and environment
Safety concerns from the additives may derive either from the active substances or from the residues of the fermentation process/production strain remaining in the final product. No concerns are expected from the fermentation process or the production strain used. Moreover, the additives show a high purity (> 99%, See Section 3.1).
Regarding the safety for the target species, the FEEDAP Panel considers that the substances per se are of no concern. l‐Glutamic acid is a naturally occurring amino acid being one of the most abundant amino acids in many plant and animal tissues. Together, with the amide glutamine, glutamic acid makes up to 10–20% of the amino acids in most proteins, and the intracellular free glutamate concentration is relatively high in many cell types. 50 A diet for chickens for fattening with about 22.4% crude protein consisting of 50% cereals (barley, maize, rye, sorghum, triticale, wheat) and 30% seed meals form (rape, soybean, sunflower) would contain about 33 g glutamic acid/kg.
Considering that the manufacturing process and production strain do not raise safety concerns and that the additives are highly purified (with < 0.1% unidentified substances), the Panel concludes that the additives are safe for the target species. The FEEDAP Panel has reservations on the use of the additives via water due to hygienic reasons (EFSA FEEDAP Panel, 2010).
Regarding the safety for consumers, l‐glutamic is ubiquitous in living organisms and is metabolised in the gastrointestinal tract of the target animals and only a very small proportion enters either the systemic or the portal blood supply. Monosodium l‐glutamate in the mammalian body is dissociated into glutamate and sodium. It is not expected that the composition of tissues and products of animal origin will be affected by the use of these additives. The FEEDAP Panel also notes that glutamic acid (E 620) and its salts (E 621 to E 625; including sodium, potassium, calcium, ammonium and magnesium salts) are included in the Union list of food additives as ‘additives other than colours and sweeteners’, ‘group I (with a maximum of 10 g/kg), ‘other additives that may be regulated combined’, category 12.1.2 salt substitutes and category 12.2.2 seasonings and condiments. 51 Considering all the above, the use of the additives under assessment in animal nutrition is considered safe for consumers.
The production strain is a genetically modified strain, but no viable cells and no DNA from the production strain were detected in the additives. Moreover, the use of l‐glutamic acid and monosodium l‐glutamate monohydrate as feed additives at the levels proposed is not expected to increase its concentration in the environment and therefore, it is of no safety concern for the environment.
3.2.2.1. Conclusions on safety for the target species, consumers and environment
The FEEDAP Panel concludes that the additives under assessment are safe for the target species, for the consumers and for the environment. However, the Panel has reservations on the use of the additives in water for drinking due to concerns on its impact on the hygienic conditions of the water.
3.2.3. Safety for user
3.2.3.1. Effects on the respiratory system
The data on dusting potential of l‐glutamic acid and one form of monosodium glutamate (See Section 3.1.3) indicate that users may be exposed by inhalation when handling the additives.
Two acute inhalation toxicity studies in rats were performed in accordance with OECD Test Guideline (TG) 403 and Good Laboratory Practice (GLP) compliant. 52 In the studies, 10 Wistar were exposed to a concentration of 5.06 g l‐glutamic acid/m3 or 5.02 g monosodium l‐glutamate monohydrate/m3 for 4 h (nose‐only). For both l‐glutamic acid and monosodium l‐glutamate monohydrate adequate mass median aerodynamic diameter of the particles in the aerosol and geometric standard deviation were achieved. At the end of the first day, most animals exposed to l‐glutamic acid displayed irregular breathing, and several animals had encrustations around the eyes and/or nose. All clinical abnormalities were fully reversible within 3 days. Soiled fur was observed in four out of five females shortly after exposure to monosodium l‐glutamate monohydrate, which was no longer seen on the next day. In four out of five male animals, irregular respiration was observed in the first days after exposure. Animals fully recovered on post‐exposure day 3 or 4. Necropsy of the animals exposed to l‐glutamic acid revealed exposure‐related red spots/patches (indicating small haemorrhages) in one or more lung lobes (in four males and three females) and one female had possibly liquid in the lungs. Necropsy of the animals exposed to monosodium glutamate monohydrate revealed exposure‐related red discolouration and/or red spots (indicating small haemorrhages) on the lungs (five males and three females), thymus (two males and three females) and mandibular lymph nodes (four males and three females). Mortality did not occur in both studies. Based on the results, it was concluded that the 4 h LC50 of l‐glutamic acid and monosodium L‐glutamate monohydrate in rats is above 5.06 g/m3 and 5.02 g/m3, respectively. Based on the observations at necropsy, the FEEDAP Panel considers the additives to be a risk by inhalation.
3.2.3.2. Effects on the skin and eyes
Two in vitro skin irritation tests conducted in accordance with OECD TG 439 and GLP were provided for the additives under assessment. 53 Based on the results obtained, l‐glutamic acid and monosodium l‐glutamate monohydrate are not irritant.
The potential of l‐glutamic acid to induce eye lesions/irritation was evaluated in an in vitro acute eye irritation study in accordance with OECD TG 438 and GLP compliant. 54 Based on the results obtained, l‐glutamic acid is not irritant to the eyes.
The potential of monosodium glutamate monohydrate, produced by fermentation with a different microbial strain but with the same specifications as the product under assessment, to cause irritation to the eyes was studied in vivo in accordance with EEC Methods for the determination of toxicity (annex to Directive 92/69/EEC), the OECD TG 405 and GLP scheme. 55 Based on the results obtained, monosodium glutamate is not irritant to the eyes.
The skin sensitisation potential of l‐glutamic acid (purity: minimum 98%) was assessed in a study conducted in accordance with OECD TG 429 and GLP compliant 56 . The additive l‐glutamic acid is not considered a skin sensitiser under the test conditions of this study. No data was provided for the skin sensitisation potential of monosodium glutamate monohydrate under the assumption that in a solution the salt would dissociate and, therefore, the results obtained with the l‐glutamic should also apply to this substance. The Panel considers that the results with l‐glutamic acid also apply to monosodium glutamate monohydrate.
3.2.3.3. Conclusions on safety for the user
Based on the studies provided, L‐glutamic acid and monosodium glutamate monohydrate are not skin or eye irritants and not skin sensitisers. The FEEDAP Panel considers that the additives to be a risk by inhalation.
3.3. Efficacy
The FEEDAP Panel considers that no data are needed to conclude on the efficacy of the substances under evaluation as nutritional additives. Nevertheless, in Appendix A, it is summarised the physiological functions of l‐glutamic acid and the data supporting the efficacy provided by the applicant. The Panel concludes that l‐glutamic acid and monosodium glutamate monohydrate are effective as amino acids for all animal species. For supplemental l‐glutamic acid and monosodium glutamate monohydrate to be as efficacious in ruminants as in non‐ruminants, it would require protection against degradation in the rumen.
l‐glutamic acid and monosodium glutamate are mentioned in Fenaroli’s Handbook of Flavour Ingredients (Burdock, 2009) and by the Flavour and Extract Manufactures Association (FEMA) as a flavour enhancer, i.e. a substance with no specific taste on its own but which has an ability to enhance existing flavours. Further, l‐glutamic acid and monosodium glutamate are authorised under Commission Regulation (EU) No 1129/2011 on food additives. The Panel considers that the effect of l‐glutamic acid and monosodium l‐glutamate monohydrate to increase the taste in food is well documented, and, therefore, no further demonstration of efficacy is necessary. The conclusions can be applied to the use in water.
3.4. Post‐market monitoring
The FEEDAP Panel considers that, for their use as nutritional additives, there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation 57 and Good Manufacturing Practice.
4. Conclusions
No viable cells of the production strain and no recombinant DNA were detected in the final additives. The use of C. glutamicum NITE BP‐01681 in the production of l‐glutamic acid and monosodium l‐glutamate monohydrate is considered safe.
Both l‐glutamic acid and monosodium glutamate monohydrate produced by C. glutamicum NITE BP‐01681 are considered to be safe for all animal species, for the consumer and for the environment. However, the use of the additive in water for drinking raises concerns for the target species due to its likely impact on the hygienic conditions of the water.
The additives l‐glutamic acid and monosodium glutamate monohydrate produced by C. glutamicum NITE BP‐01681 are considered not irritant to skin or eyes and not a dermal sensitiser. However, they are considered a risk by inhalation.
The use of l‐glutamic acid and monosodium glutamate monohydrate produced using C. glutamicum NITE BP‐01681 can be efficacious as nutritional additives or as flavouring compounds.
5. Documentation as provided to EFSA/Chronology
| Event | |
|---|---|
| 18/06/2020 | Dossier received by EFSA. FAD‐2020‐0047. Submitted by Ajinomoto Animal Nutrition Europe. |
| 07/07/2020 | Reception mandate from the European Commission |
| 13/11/2020 | Application validated by EFSA – Start of the scientific assessment |
| 12/02/2021 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 15/02/2021 | Comments received from Member States |
| 17/03/2021 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation and user safety |
| 13/01/2022 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 26/01/2022 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AA
Amino acids
- CAA
conditionally essential amino acids
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- DM
dry matter
- EAA
essential amino acids
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- LOQ
Limit of quantification
- NEAA
non‐essential amino acids
Appendix A – Physiological functions of Glutamic acid
Amino acids (AA) have a significant physiological relevance. They serve as components of proteins and substrates for synthesis of low‐molecular‐weight substances. Based on growth, milk and egg production or nitrogen balance of animals, AA were traditionally classified as nutritionally essential (EAA) or non‐essential (dispensable; NEAA). Principally, EAA cannot be synthesised in the body, they must be administered to the animal usually via feed. Some NEAA are also known as conditionally essential (CEAA) – earlier called semi‐essential – when in case of a particularly high or specific requirement (e.g. growth of young animals, stress), this cannot be fully covered by body’s own synthesis. NEAA have been ignored for a long time in the nutrition of all species. Glutamic acid is not an essential amino acid as no nutritional requirements have been described for poultry, pigs, ruminants, cats, dogs, horses, fish or crustaceans.
However, it was already demonstrated in the 1960s and 1970s that non‐specific amino‐nitrogen is essential for optimal growth and nitrogen deposition in growing chicks, piglets and, to a minor extent, in rats. The supply of nonspecific amino‐N and the EAA/NEAA ratio are evidently more important in young animals fed protein‐reduced diets, partially because of the inefficient synthesis and limited capacity to synthesise dispensable AA and, at the same time, an increasing demand for NEAA synthesis.
Considering the high abundance of Glu in feed materials, it seems unlikely that Glu deficiencies may occur under conventional feeding conditions. However, Glu supplementation might have to be considered when protein‐reduced diets are fed (lower N output for sustainable agriculture) and the ideal protein concept demands for a considerable supplementation of the most limiting AA (in case of piglets: lysine, methionine, tryptophan, threonine and possibly arginine), so that NEAA, and particularly Glu, become scarce. Roth et al. (1994) showed that the supply of Ala, Asp, Gly and Ser is not necessary for piglets fed crystalline AA diets since the omission of one of these AA did not affect the N balance parameters, whereas diets lacking Glu tended to reduce the N retention (by 6%) and significantly impaired N utilisation (by 7%).
Key data in support of the effect of Glu in protein‐reduced diets were published by Schumacher (2002). Piglets were fed protein‐reduced diets (14.5% and 16.6% CP; soy/grain based) with graded levels of Glu (1.7–5.4%) and EAA/NEAA ratios between 69:31 and 54:46. Glu addition significantly improved N retention and N utilisation with a significant positive linear relationship (p < 0.0001) between Glu intake and N retention.
Schuhmacher (2002) further suggested, considering another study with a more drastic protein reduction, that EAA/NEAA ratios higher than 54:46 (Arg considered as EAA) may adversely affect N retention and utilisation in young pigs fed protein‐reduced diets. Roth et al. (1993) concluded approximately the same ratio (53:47) as optimal for growing pigs. 58 Similar examples for other species can be found in other works. 59
There is evidence that all preformed AA are needed, not only for monogastric animals but also for high‐producing cows and rapidly growing ruminants. Many NEAA and CEAA (e.g. Arg, Gln, Glu, Gly and Pro) and certain EAA (e.g. Leu and Trp) participate in cell signalling, gene expression and metabolic regulation.50
When formulating optimal diets and considering a potential Glu supplementation, an adequate EAA to NEAA ratio as well as functions beyond protein synthesis should be considered.
The below information supporting the efficacy is mainly based on a review provided by the applicant summarising the metabolism and nutritional effects of glutamic acid mainly in poultry, pigs, ruminants and fish. 60 The applicant also conducted a literature review 61 focusing on the effects of l‐glutamic acid and monosodium glutamate in pigs and chickens for fattening. However, most of the publications retrieved were already considered in the first above‐mentioned review, or provided no relevant additional information.
Dietary glutamate in pigs
Recent studies have shown that: (i) dietary Glu is a major energy substrate in the small intestine of pigs and (ii) sufficient provision of dietary Glu can enhance villus height and whole‐body growth in weanling pigs. Furthermore, Glu can improve barrier and antioxidative functions in porcine small intestinal epithelial cells. Similarly, dietary supplementation with Glu plus aspartate can alleviate oxidative stress in weaned piglets challenged with hydrogen peroxide. To date, provision of dietary Glu is particularly important in the pig industry, because low‐protein diets, which are currently used to reduce the production of nitrogenous wastes by swine farms, do not sufficiently supply Glu or its AA precursors. Consequently, Glu is considered a CEAA for pigs.
Dietary glutamate in ruminants
Pre‐ruminants have similar patterns of Glu utilisation and metabolism to non‐ruminant animals. There is evidence that pre‐ruminants, as well as sheep, goats and beef cattle, require Glu supplementation for: (i) maximal growth and production performance and (ii) optimal intestinal health and function. However, in contrast to non‐ruminants, ruminants (e.g. sheep and cattle) extensively utilise dietary protein‐bound AA, including Glu, in the rumen for the synthesis of microbial protein. Thus, in post‐weaning ruminants, some of the dietary protein‐bound Glu may not enter the lumen of the small intestine and the portal vein.
Dietary glutamate in poultry
Chickens grow very rapidly and respond sensitively to dietary AA intake. Glu has long been used to provide nitrogen and carbon sources for the synthesis of AA in chicks. Importantly, Glu has often been used to balance dietary nitrogen content in studies involving the development of ‘ideal protein’ in chicken nutrition. The review refers to 10 studies in chickens for fattening and one in laying hens. Most chicken data indicate better growth and feed to gain ratio (n = 7), in laying hens egg production increased without influence on egg mass.
Dietary glutamate in fish
Relatively little is known about cell‐specific metabolism and nutrition of Glu in fish. A total of six studies showed beneficial effects of dietary Glu in fish: improvement of fillet quality, antioxidative capacity and lean tissue growth in Atlantic salmon; of protein retention, body growth and feed to gain ratio in Gilthead seabream; of fillet quality and hepatic fat deposition (decreased) in Atlantic cod; of digestive function and body growth in grass carp; and of growth and intestinal villus height in rainbow trout.
Considering all the information above, l‐glutamic acid and monosodium glutamate monohydrate can be regarded as effective amino acids for all animal species. For supplemental l‐glutamic acid and monosodium glutamate monohydrate to be as efficacious in ruminants as in non‐ruminants, it would require protection against degradation in the rumen.
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for l‐Glutamic acid and Monosodium l‐glutamate monohydrate produced using strain Corynebacterium glutamicum NITE BP‐01681
In the current application, an authorisation is sought under Article 4(1) for l‐glutamic acid and monosodium l‐glutamate monohydrate produced using the strain Corynebacterium glutamicum NITE BP‐01681, under the categories/functional groups 2(b) ‘sensory additives'/‘flavouring compounds' and 3c ‘nutritional additives'/‘amino acids, their salts and analogues', according to Annex I of Regulation (EC) No 1831/2003. The authorisation is sought for all animal species.
According to the Applicant, the feed additives (l‐glutamic acid and monosodium l‐glutamate monohydrate) are white crystalline powders with a minimum purity (mass fraction) of 98% (based on anhydrous weight). The feed additives are intended to be added directly into feedingstuffs or through premixtures and water for drinking. The Applicant proposed no minimum or maximum content of l‐glutamic acid and monosodium l‐glutamate monohydrate in feedingstuffs when used as nutritional additives, while inclusion levels of up to 10 g of l‐glutamic acid and monosodium l‐glutamate monohydrate/kg feedingstuffs were suggested by the Applicant when used as sensory additives.
For the quantification of glutamic acid and monosodium glutamate in the feed additives and premixtures, the Applicant submitted the ring‐trial validated method EN ISO 17180:2013 dedicated for the determination of lysine, methionine and threonine in commercial amino acid products and premixtures containing more than 10% of amino acid. The method does not distinguish between the amino acids and their salts, or between different salts of the same amino acids, and it cannot differentiate between enantiomers. The Applicant presented the results from validation and verification studies demonstrating the extension of scope of the above‐mentioned method for the determination of glutamic acid and monosodium glutamate in the feed additives and premixtures. The following performance characteristics were reported for the determination of glutamic acid and monosodium glutamate in the feed additives and premixtures: a relative standard deviation for repeatability (RSDr) ranging from 0.3 to 5.3%, a relative standard deviation for intermediate precision (RSDip) ranging from 0.3 to 5.6% and a recovery rate (RRec) ranging from 97 to 103%.
Based on the performance characteristics available, the EURL recommends for official control the ring‐trial validated method EN ISO 17180:2013 based on IEC‐VIS/FLD for the quantification of glutamic acid and monosodium glutamate in the feed additives and premixtures.
For the quantification of glutamic acid and monosodium glutamate content in feedingstuffs, the Applicant submitted the ring‐trial validated European Union (EU) method (Commission Regulation (EC) No 152/2009) based on IEC with photometric detection (VIS). This method, designed for the analysis of amino acids in premixtures and feedingstuffs, does not distinguish between the amino acids and their salts or between different salts of the same amino acids and it cannot differentiate between enantiomers.
The EU method was further ring‐trial validated resulting in the EN ISO 13903:2005 method. The following performance characteristics were reported for the quantification of glutamic acid in feed at mass fractions ranging from 15.1 to 79.7 g/kg: RSDr ranging from 0.9 to 2.7% and a relative standard deviation for reproducibility (RSDR) ranging from 4.7 to 9.1%. Furthermore, a limit of quantification (LOQ) ranging from 30 to 350 mg/kg has been reported for the analysis of various amino acids, while a specific LOQ for glutamic acid (or monosodium glutamate) has not been indicated.
In addition, based on NRL's experience and following the previous reports for various amino acids, the EURL recently recommended in the frame of another evaluation report for monosodium l‐glutamate (FAD‐2018‐0090) the above‐mentioned EU method for the quantification of monosodium glutamate in the feed additive.
Based on the overall available data, the EURL recommends for official control the ring‐trial validated EU method, based on IEC‐VIS to quantify glutamic acid and monosodium glutamate in the feed additives, premixtures and feedingstuffs (only when they are intended to be used as nutritional feed additives).
Since it is not known what will be the maximum recommended content authorised for glutamic acid and monosodium glutamate in feedingstuffs when they are used as sensory additives/flavouring compounds in the frame of the current dossier, the EURL is unable to recommend the EU method for the official control of glutamic acid and monosodium glutamate in feedingstuffs when they are intended to be used as sensory additives. However, the EU method is at least fit‐for‐purpose for the quantification of glutamic acid and monosodium glutamate in feed in the validated concentration range.
For the quantification of glutamic acid in water, the Applicant submitted the ring‐trial validated EN ISO 13903:2005 method, which is equivalent to above‐mentioned EU method. This method was successfully applied in the frame of the stability studies of glutamic acid in water. Hence, the EURL recommends for official control the above‐mentioned EU method based on IEC‐VIS to quantify glutamic acid and monosodium glutamate in water.
For the identification of the feed additives, the EURL recommends the ‘l‐Glutamic acid’ and ‘Monosodium l‐glutamate’ monographs of the Food Chemical Codex (FCC).
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Gropp J, Herman L, Tosti L, Galobart J, Pizzo F, Revez J and Anguita M, 2022. Scientific Opinion on the safety and efficacy of the feed additives consisting of l‐glutamic acid and monosodium l‐glutamate monohydrate produced by Corynebacterium glutamicum NITE BP‐01681 for all animal species (METEX NOOVISTAGO). EFSA Journal 2022;20(3):7156, 19 pp. 10.2903/j.efsa.2022.7156
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00502
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to acknowledge the contribution to this opinion by the Working Groups on Animal Nutrition, Microbiology and Toxicology as well as Elisa Pettenati, Paola Manini and Jordi Ortuño.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 26 January 2022
Notes
REGULATION (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Ajinomoto Animal Nutrition Europe, Rue Guersant 32, 75017 Paris, France. In July 2021 EFSA was informed on the change in the Name of the Applicant/Company from Ajinomoto Animal Nutrition Europe to METEX NOOVISTAGO.
FEED dossier reference: FAD‐2020‐0047.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep‐fad‐2020‐0047‐glutamic‐acid‐glutamate.pdf
COMMISSION REGULATION (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annexes Sect II Supp/Annex II.1.
Technical dossier/Section II/Annexes Section II Supp/Annexes II.2, II.3 and Supplementary information January 2022/230721.
Technical dossier/Section II/Annexes Section II Supp/Annex II.4 and II.5.
Technical dossier/Section II/Annexes Section II Supp/Annex II.3.
Technical dossier/Section II/Annexes Sect II Supp/Annex II.2
Technical dossier/Section II/Annexes Section II Supp/Annex II.22.
Technical dossier/Section II/Annex II.8 and Annex II.13. ■■■■■
Technical dossier/Section II/Annex II.8.
COMMISSION REGULATION (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to REGULATION (EC) No 1333/2008 of the European Parliament and of the Council (Text with EEA relevance). OJ L 83, 22.3.2012, p. 1.
Technical dossier/Section II/Annex II.8 and Annex II.9.
Technical dossier/Section II/Annex II.9 ■■■■■
Technical dossier/Section II/Annex II.9 and II.25 LOQ in mg/kg was 0.005 for mercury, 0.01 for cadmium, 0.05 for lead and melamine, 0.1 for arsenic, 1.5 for hydrocyanic acid and 20 for fluorine. For the mycotoxins (in µg/kg) was 0.1 for aflatoxins B1, B2 and G1, 0.2 for aflatoxin G2 and ochratoxin A, 20 for zearalenone, T‐2 toxin, HT‐2 toxin and fumonisins B1, B2 and B3, and 50 for deoxynivalenol.
Technical dossier/Section II/Annex II.9. LOQ in mg/kg ranged 0.002–0.1 for organochlorine pesticides (including pyrethroids), and from 0.01 to 0.03 for organophosphorus pesticides (depending on the parameter analysed).
Technical dossier/Section II/Annex II.9.
Technical dossier/Section II/Annex II.11.
Technical dossier/Section II/Annex II.12.
Technical dossier/Section II/Annexes Section II Supp/Annex II.34.
Technical dossier/Section II/Annexes Section II Supp/Annex II.36 and Supplementary information January 2022/230721.
Technical dossier/Section II/Annex II.42.
Technical dossier/Section II/Annex II.10.
Technical dossier/Section II/Annex II.14. ■■■■■
Technical dossier/Supplementary information January 2022/130122/Annex 5.
Technical dossier/Section II/Annex II.14.
Technical dossier/Section II/Annex II.14 and 15.
Technical dossier/Section II/Annex II.15 ■■■■■
Technical dossier/Section II/Annex II.15.
Technical dossier/Section II/Annex II.15. LOQ in mg/kg was 0.005 for mercury, 0.01 for cadmium, 0.05 for lead and melamine, 0.1 for arsenic, 1.5 for hydrocyanic acid and 20 for fluorine, for the mycotoxins (in µg/kg) was 0.1 for aflatoxins B1, B2 and G1, 0.2 for aflatoxin G2 and ochratoxin A, 20 for zearalenone, T‐2 toxin, HT‐2 toxin and fumonisins B1, B2 and B3, and 50 for deoxynivalenol.
Technical dossier/Section II/Annex II.15. LOQ in mg/kg ranged 0.002 to 0.1 for organochlorine pesticides (including pyrethroids), and from 0.01 to 0.03 for organophosphorous pesticides (depending on the parameter analysed).
Technical dossier/Section II/Annex II.17.
Technical dossier/Section II/Annex II.18.
Technical dossier/Section II/ Annexes Section II Supp/Annex II.35.
Technical dossier/Section II/ Annexes Section II Supp/Annex II.37 and Supplementary information January 2022/230721.
Technical dossier/Section II/Annex II.43.
Technical dossier/Section II/Annex II.16 and Supplementary information January 2022/301121_Annex Supp_Info2_An_4.
Technical dossier/Section II/Annex II.63.
Technical dossier/Supplementary information January 2022/230721/Annex 3.
Technical dossier/Supplementary information January 2022/230721/Annex 4.
Technical dossier/Section II/Annex II.63 and Supplementary information January 2022/230721/Annex 3.
Technical dossier/Section II/Annex II.67.
Technical dossier/Section II/Annex II.69.
Technical dossier/Section II/Annex II.71.
The applicant provided data on the stability of the active substance in a food sauce only which was not further considered/Supplementary information January 2022/230721/Annex 5.
Technical dossier/Section III/Annex III.1.
COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives Text with EEA relevance OJ L 295, 12.11.2011, p. 1.
Technical dossier/Section III/Annex III 15 and Annex III 16.
Technical dossier/Section III/Annex III 19 and Annex III 20.
Technical dossier/Section III/Annex III 17.
Technical dossier/Section III/Annex III 18 and supplementary information January 2022/301121/Annex 6.
Technical dossier/Section III/Annex III 21.
REGULATION (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
Roth et al. (1993) proposed 47:53 when attributing arginine to the NEAA. The value given here (53:47) is recalculated considering arginine as essential for this life period of pigs.
Technical dossier/Section III.
Technical dossier/Section III/Annex III.1 and Section IV.
Technical dossier/Section IV.
References
- Burdock GA, 2009. Fenaroli’s Handbook of Flavor Ingredients, 6th Edition. CRC Press. Taylor & Francis Group, Boca Raton, FL. 757–758, 1141–1142. 10.1201/9781439847503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(10):587, 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernández Escámez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S and Herman L, 2020. Scientific Opinion on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA Journal 2020;18(2):5966, 56 pp. 10.2903/j.efsa.2020.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances Used in Animal Feed) , 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth FX, Markert W and Kirchgessner M, 1993. Zur optimalen Versorgung mit α‐Aminostickstoff von Mastschweinen. Journal of Animal Physiology and Animal Nutrition, 70, 196–206. 10.1111/j.1439-0396.1993.tb00323.x [DOI] [Google Scholar]
- Roth FX, Fickler J and Kirchgessner M, 1994. N‐Bilanz von Ferkeln bei vollständigem Fehlen von einzelnen nicht‐essentiellen Aminosäuren im Futter. 2. Mitteilung zur Bedeutung nicht‐essentieller Aminosäuren für den Proteinansatz. Journal of Animal Physiology and Animal Nutrition, 72, 215–224. 10.1111/j.1439-0396.1994.tb00390.x [DOI] [Google Scholar]
- Schuhmacher A, 2002. Limitierende Aminosäuren im Futter für wachsende Schweine Habilitationsschrift, Universität Leipzig, Veterinärmedizinische Fakultät. [Google Scholar]


