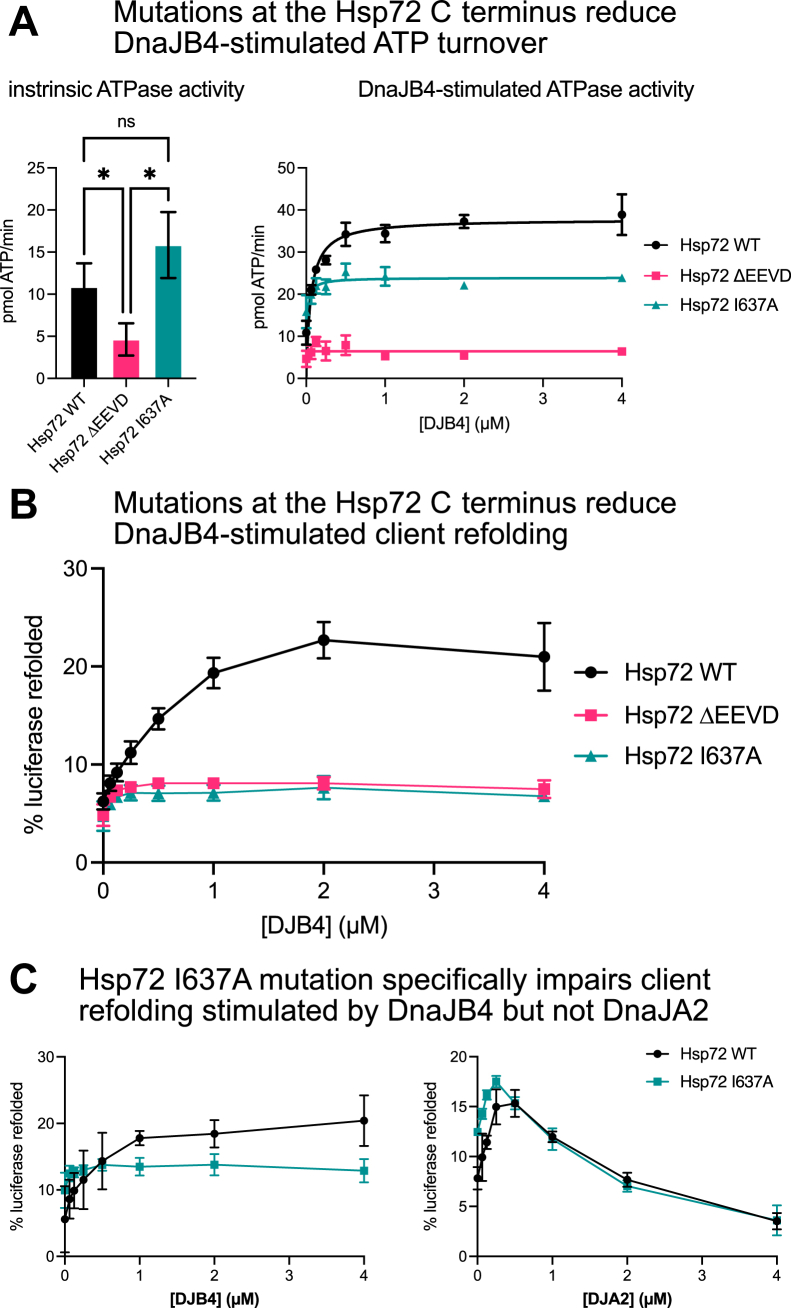
Figure 6.
Mutations in the EEVD motif reduce collaboration between Hsp72 and DnaJB4.A, ATP hydrolysis assay comparing the turnover rate of Hsp72 WT and mutants in the presence of DnaJB4, as measured by malachite green assay. The left graph shows mean intrinsic ATPase rate ±SD (n = 3) of the various Hsp72 mutants. The right graph shows mean ATPase rate ±SD (n = 3) of the various Hsp72 mutants in the presence of increasing concentrations of DnaJB4. Curves were fit according to Michaelis–Menten kinetics at steady state. Statistics were performed using unpaired Student’s t test (∗p < 0.05, ns, not significant). B, luciferase refolding assay comparing WT and mutant Hsp72 in the presence of DnaJB4. Refolding was measured by SteadyGlo luciferase reagent (see the Experimental procedures). The graph shows mean percent luciferase refolded relative to nondenatured luciferase control ±SD (n = 3). C, luciferase refolding assay comparing WT and I637A mutant Hsp72 in the presence of DnaJB4 or DnaJA2. The graph shows mean percent luciferase refolded relative to nondenatured luciferase control ±SD (n = 3).