Figure 7.
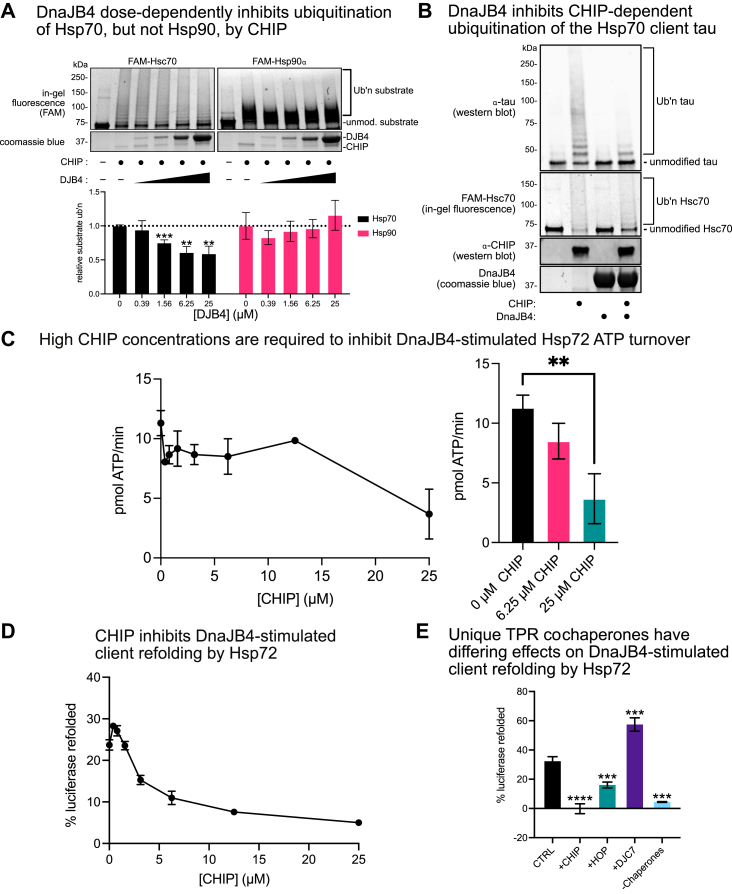
Competition for the EEVD motif by cochaperones regulates chaperone functions.A, in vitro ubiquitination assay comparing ubiquitination of FAM-labeled Hsc70 or Hsp90⍺ by CHIP in the presence of increasing amounts of DnaJB4. Samples were separated by SDS-PAGE, and ubiquitination was analyzed by in-gel fluorescence, whereas CHIP and DnaJB4 were identified by staining with Coomassie blue. The graph shows mean substrate ubiquitination relative to no DnaJB4 control ±SD (n = 3). Statistics were performed using unpaired Student’s t test (∗∗p < 0.01, ∗∗∗p < 0.001 compared with no DnaJB4 control). B, in vitro ubiquitination assay comparing ubiquitination of MAPT/tau by CHIP in the presence of DnaJB4. Hsc70 was identified by in-gel fluorescence, whereas DnaJB4 was identified by staining with Coomassie blue. CHIP and tau were identified by Western blot. C, malachite green ATP hydrolysis assay comparing ATP turnover rate of WT Hsp72 in the presence of constant DnaJB4 and increasing concentrations of CHIP. Graph shows mean ATP hydrolysis rate ±SD (n = 3). Statistical analysis in the right panel was performed using unpaired Student’s t test (∗∗p < 0.01). D, luciferase refolding assay comparing ability of Hsp72 to refold client in the presence of DnaJB4 and CHIP. The graph shows mean percent luciferase refolded relative to nondenatured luciferase control ±SD (n = 3). E, luciferase refolding assay comparing the ability of Hsp72/DnaJB4 to refold client in the presence of various tetratricopeptide repeat (TPR) cochaperones. The graph shows mean percent luciferase refolded relative to non-denatured luciferase control ±SD (n = 3).