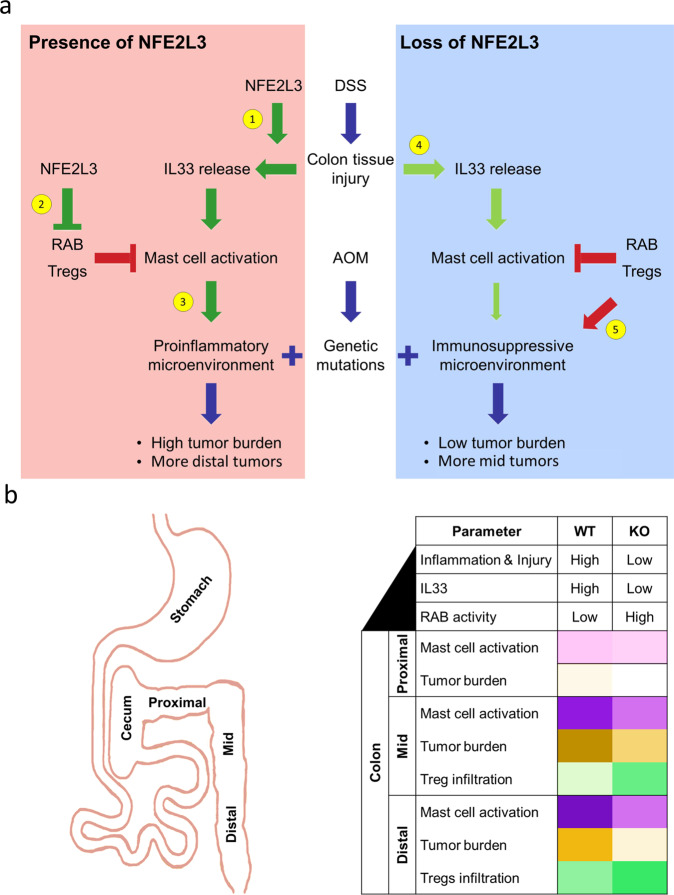
Fig. 6. Role of the NFE2L3 transcription factor in colitis-induced CRC.
a Model of the regulation of CRC development in the presence and absence of NFE2L3. AOM/DSS induces colonic epithelial tissue damage resulting in the release of the alarmin IL33. 1) NFE2L3 promotes IL33 expression thus leading to mast cell activation; 2) NFE2L3 inhibits Tregs and expression of RAB pathway transcripts hence permitting mast cell activation and degranulation; 3) Elevated mast cell activity led by NFE2L3 expression promotes a proinflammatory microenvironment leading to a higher tumor burden and enrichment of tumors in the distal colon; 4) In absence of NFE2L3, the colonic tissue releases less IL33 in response to injury leading to reduced mast cell recruitment. 5) In the absence of NFE2L3, RAB pathway members and Tregs can inhibit mast cell activity hence promoting an immunosuppressive microenvironment characterized by a lower tumor burden and proximal sided tumors. b Table summarizing the differences between wildtype and knockout CRC tissues. The colons of Nfe2l3 knockout mice display less inflammation and a lower number of tumors when compared to their wild type littermates. Tumors are significantly enriched in the mid versus the distal section of the colon. In addition, Nfe2l3−/− mice exhibit reduced mast cell infiltration mostly in the distal section of the colon. Finally, knockout mouse colons display significantly higher infiltration by Tregs particularly in the mid section of the colon.