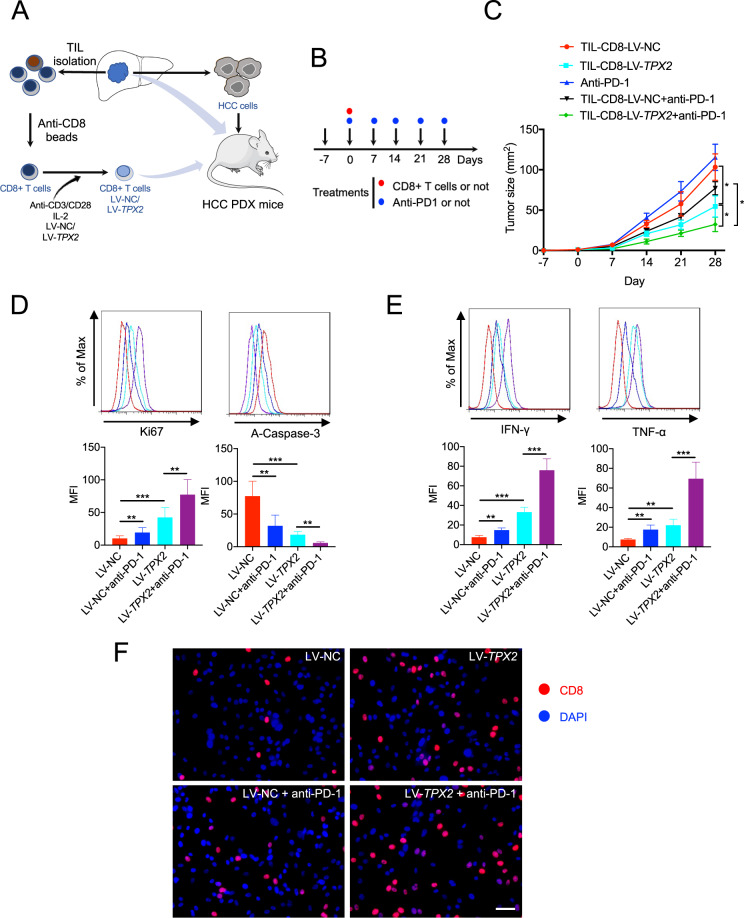
Fig. 4. TPX2 overexpression delayed tumor growth and increased the antitumor function and anti-PD-1 response of CD8+ T cells in an HCC PDX mouse model (subcutaneous tumor-bearing model).
A Illustration of HCC PDX mouse model construction and adoptive T cell transfer for therapeutic purposes. B Illustration of CD8 + T cell transfer (i.v. 2 × 106 cells) and/or anti-PD-1 treatment (i.v.) at the indicated time points. Day -7 indicates the first day of human HCC cell inoculation. C Tumor growth was observed weekly in human HCC-bearing NSG mice (that received human CD8 + T cells or anti-PD-1 treatment) (n = 5). D, E Thirty days after Hepa1-6 cell inoculation, the tumors were removed for TIL isolation. Proliferation (Ki67), apoptosis (A-Caspase-3) (D), and the production of effector cytokines (IFN-γ and TNF-α) (E) were measured. F CD8 + T cell staining in tumors sections. Scar bar: 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001; the two-tailed unpaired Student’s t-test was used to compare two groups. A-Caspase-3 activated Caspase 3, anti-PD-1 antagonist antibody targeting PD-1, HCC hepatocellular carcinoma, IFN-γ interferon gamma, MKI67 Marker of proliferation Ki67, LV-TPX2 lentivirus used to overexpress the human TPX2 gene, MFI mean fluorescence intensity, LV-NC lentivirus used as a control, PDX patient-derived xenograft, TNF-α tumor necrosis factor alpha, TIL tumor-infiltrating lymphocyte.