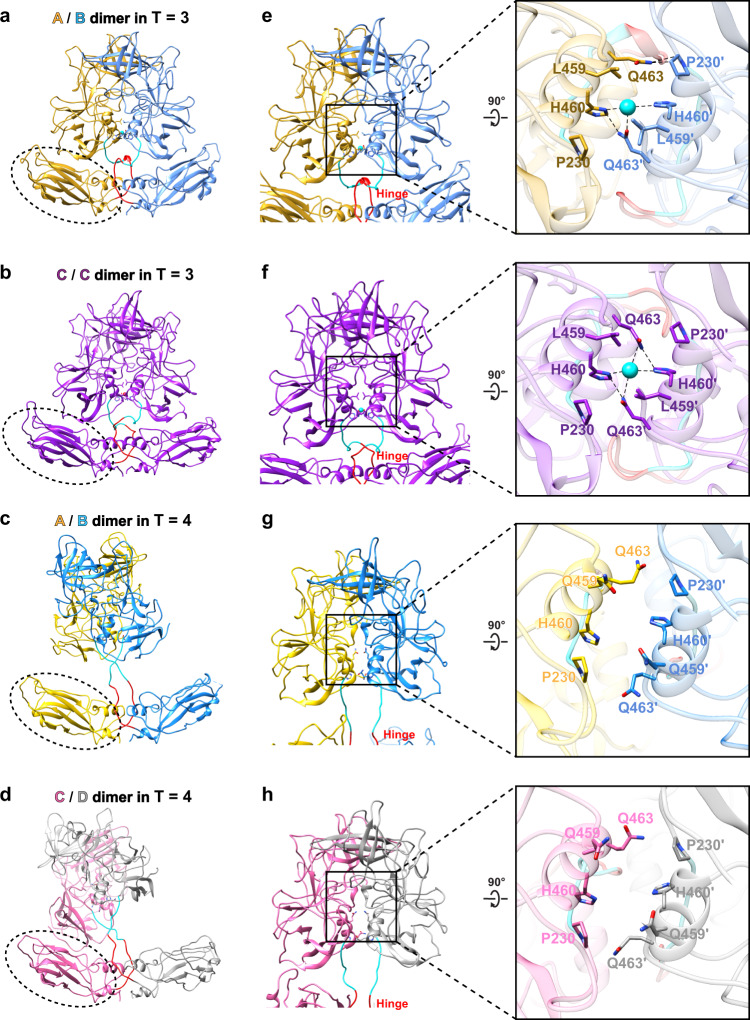
Fig. 7. Comparison of different quasiequivalent VP1 dimers in the resting conformation in T = 3 HOV capsid vs the raised conformation in T = 4 Minerva capsid.
Comparison of the dimers using the S domain (shown in dashed oval) (left panel, a–d), or the P domain (middle panel, e–h) for structural alignment. The additional C/D dimer present in the T = 4 capsid is shown in the bottom row (d, h). The inset (right panel) corresponding the the boxed region in the middle panel shows a close up view of the interactions at the dimeric interface viewed along the two-fold axis of the P dimer. The ion present only in the T = 3 dimers is shown as a cyan sphere. The A, B, C, and D subunits are labeled, and colored in yellow, blue, purple, and gray respectively (the C subunit of the T = 4 C/D dimer is shown in lighter shade of purple to distinguish it from the C subunits of C/C dimer)). Residues 213-221 (hinge) and 222-229 (N-terminus of P domain) are colored red and cyan, respectively.