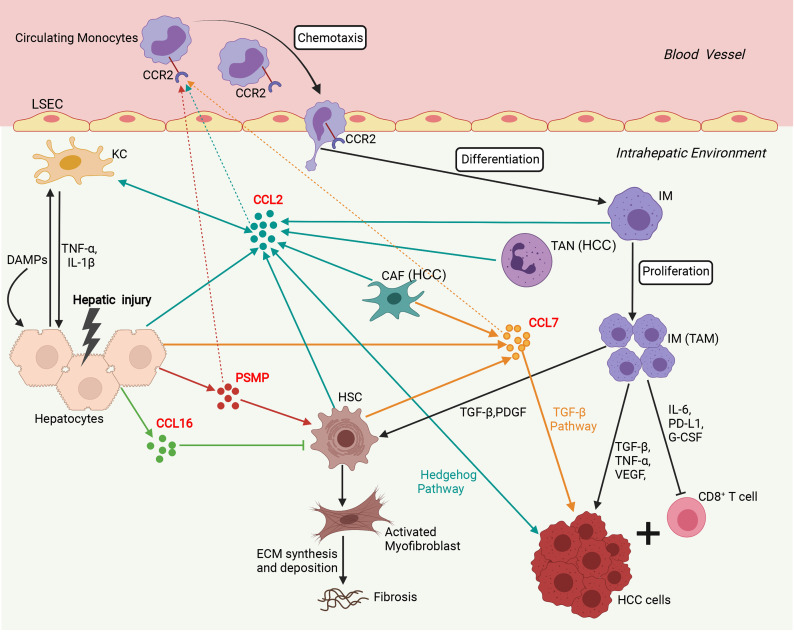
Figure 2.
Involvement of the network of CCR2 and its ligands in regulation of immune mechanisms during liver injury, fibrosis and hepatocarcinogenesis. Sophisticated experimental mouse models of liver injury, fibrosis, and HCC revealed the complex interplay of different chemokines and liver cells. In the initial phase, upon hepatocyte injury, DAMPs activate KCs that in turn secrete inflammatory cytokines (TNF-α, IL-1β), which contribute to further hepatocyte injury and release chemokines including, CCL2, CCL7, CCL16, and PSMP. CCL2, CCL7, and PSMP promote the recruitment of CCR2+ circulating monocytes into the injured liver, where they develop into inflammatory, angiogenic, and fibrogenic IMs. During chronic injury, these macrophages activate HSCs by secreting TGF-β and PDGF to become collagen-producing myofibroblasts responsible for excessive extracellular matrix (ECM) synthesis and deposition, promoting liver fibrosis development. In addition, CCL16 directly inhibits the activation of HSCs, and PSMP directly promotes the activation of HSCs during liver fibrosis. In the HCC tumor microenvironment, TANs and CAFs could recruit TAMs by secreting CCL2 and CCL7. TAMs, specifically the M2 phenotype, promoted tumor growth, invasiveness, and metastasis by suppressing CD8+ T lymphocyte responses and producing PD-L1, IL-6, G-CSF, TNF-α, TGF-β, and VEGF. Additionally, CCL2 could directly promote HCC progression through activation of the Hh pathway. CCL7 could directly enhance the mesenchymal phenotype of HCC cells and facilitate their migration and invasion through the TGF-β signaling pathway. CAF, cancer-associated fibroblast; DAMP, danger-associated molecular pattern; ECM, extracellular matrix; G-CSF, granulocyte-colony stimulating factor; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; IM, infiltrating macrophage; KC, Kupffer cell; LSEC, liver sinusoidal endothelial cell; PDGF, platelet-derived growth factor; PD-L1, programmed death-ligand 1; PSMP, PC3-secreted microprotein; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; TNF-α, tissue necrosis factor α; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor. (Figure created with BioRender.com).