Abstract
Climate change poses a major threat to coral reefs. We conducted an outdoor 22-month experiment to investigate if coral could not just survive, but also physiologically cope, with chronic ocean warming and acidification conditions expected later this century under the Paris Climate Agreement. We recorded survivorship and measured eleven phenotypic traits to evaluate the holobiont responses of Hawaiian coral: color, Symbiodiniaceae density, calcification, photosynthesis, respiration, total organic carbon flux, carbon budget, biomass, lipids, protein, and maximum Artemia capture rate. Survivorship was lowest in Montipora capitata and only some survivors were able to meet metabolic demand and physiologically cope with future ocean conditions. Most M. capitata survivors bleached through loss of chlorophyll pigments and simultaneously experienced increased respiration rates and negative carbon budgets due to a 236% increase in total organic carbon losses under combined future ocean conditions. Porites compressa and Porites lobata had the highest survivorship and coped well under future ocean conditions with positive calcification and increased biomass, maintenance of lipids, and the capacity to exceed their metabolic demand through photosynthesis and heterotrophy. Thus, our findings show that significant biological diversity within resilient corals like Porites, and some genotypes of sensitive species, will persist this century provided atmospheric carbon dioxide levels are controlled. Since Porites corals are ubiquitous throughout the world’s oceans and often major reef builders, the persistence of this resilient genus provides hope for future reef ecosystem function globally.
Subject terms: Climate-change ecology, Ecophysiology, Marine biology, Climate-change impacts
Introduction
Coral reefs are threatened worldwide due to the environmental impacts of climate change1. Rising seawater temperature due to global warming is considered to be the greatest threat to corals as it induces mass coral bleaching within and across tropical reef regions2–4. Thermal stress during ocean warming events causes a breakdown of the coral-algal symbiosis, and as a result, the algal endosymbionts (family Symbiodiniaceae) are expelled leaving corals white in color and unable to obtain fixed carbon through photosynthesis5,6. If thermal stress is prolonged, corals are unable to recover their Symbiodiniaceae partners which can lead to decreases in coral health, increases in disease prevalence, and high levels of coral mortality7,8. The second global threat to coral reefs is ocean acidification. Reduced seawater pH can cause dissolution and weakening of coral skeletons, has been shown to slow or even stop calcification, and can also exacerbate the negative effects of temperature stress on some species (e.g.,9–12). Overall, it is predicted that seawater temperature will increase by another 0.2–3.5 °C with concomitant drops in pH of 0.1–0.3 units by the year 2100, depending on the CO2 emissions scenario13.
While the response of corals to future ocean stress of elevated temperature coupled with reduced pH has been the focus of a growing number of studies over the last decade (e.g.,12,14,15), the physiological traits that allow the coral holobiont (i.e., animal host, algal endosymbionts, and associated microbiome) to cope with multi-annual exposure to baseline shifts in both stressors, coupled with repeat summer heat-wave events as is expected later this century, is unknown. Here, we define holobiont “coping” as survival under the dual stress of ocean warming and acidification conditions, coupled with the maintenance of coral pigmentation, net positive calcification, maintenance of tissue biomass and energy reserves, and sufficient fixed carbon acquisition through photosynthesis and heterotrophy to meet metabolic demand. These traits were selected as an increase in coral whiteness is a visible sign of bleaching due to loss of chlorophyll and Symbiodiniaceae cells16,17. Patterns in calcification rates and tissue biomass are indicative of energy allocation between skeletal structures and somatic tissue18. Energy reserves are known to be important indicators of coral health19–22 which corals rely upon during times of metabolic energy deficits23,24. Heterotrophy is critical to coral lipid synthesis and tissue rebuilding following bleaching25,26. Finally, the inability of corals to meet metabolic demand is a clear indicator of stress27–29. Here we address two overarching questions: (1) Which corals will survive chronic future ocean conditions? and (2) How well do survivors cope with future ocean conditions? We investigated three of the most abundant coral species in Hawaiʻi (Montipora capitata, Porites compressa, and Porites lobata). These species experience differential resilience to thermal stress and/or ocean acidification (e.g.,14,30,31) and varying capacities to recover from bleaching (e.g.,26,28), making them ideal candidates to evaluate the likely responses of Hawaiian corals to future ocean conditions.
Methods
Coral species, sample collection, and acclimation
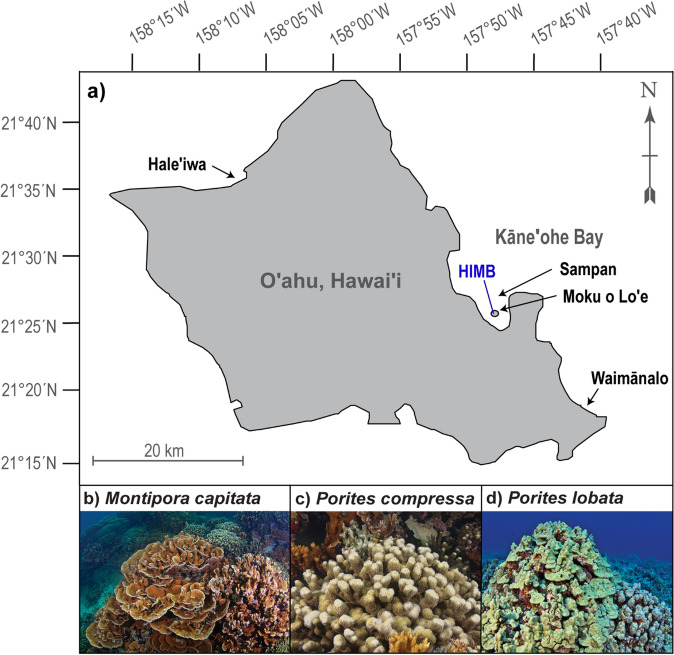
The corals Montipora capitata (branching and encrusting), Porites compressa (branching), and Porites lobata (massive) were collected at 2 ± 1 m depth between 29 August and 11 November 2015 from four reef sites around the island of Oʻahu, Hawaiʻi: Moku o Loʻe and Sampan within Kāneʻohe Bay, Waimānalo, and Haleʻiwa (Fig. 1). P. lobata was not found at Moku o Loʻe and was not collected there. This broad spatial sampling of corals helped to ensure that a representative sample of the genetic variation present in these species from Oʻahu was included in the study. Six genets of each species were collected at each site using a hammer and chisel for a total of 66 genets (24 parent colonies for M. capitata, 24 parent colonies for P. compressa, and 18 parent colonies for P. lobata) (Table S1). Species-specific microsatellite markers (developed by32,33) were used to genotype all corals and ensure that they were genetically distinct. After removal from the reef, genets were placed in individual plastic bags filled with seawater from the collection site, and transported back to the Hawaiʻi Institute of Marine Biology (HIMB, Fig. 1a). Four clonal ramets were cut from each genet using a band saw, and each ramet was mounted on a labelled ceramic plug using cyanoacrylate gel. The 264 ramets (i.e., 66 genets × 4 ramets, Table S1) were distributed among the experimental outdoor flow-through mesocosm tanks, and allowed to recover and acclimate to the mesocosm system under ambient reef-supplied flow-through seawater for at least 12 weeks until 31 January 2016. Shade cloth above the mesocosm tanks attenuated sunlight by 30% to provide irradiance like that at collection depth, with a maximum instantaneous irradiance of ~ 1730 µmol m−2 s−134. A complete timeline of experimental procedures can be found in Fig. S1.
Figure 1.
(a) Map of four coral collection sites around the island of Oʻahu, Hawaiʻi (black text), experimental location at the Hawaiʻi Institute of Marine Biology (HIMB, blue text), and photos of the study species (b) M. capitata, (c) P. compressa, and (d) P. lobata. Photographs courtesy of Keoki Stender.
Mesocosm experiment
This study was part of a larger mesocosm experiment that is comprehensively described previously35. Meta-data regarding the experimental methods can be found in Table S2 and a detailed description of the experimental methods are provided in the Supplemental Text. Briefly, the experiment consisted of four treatments (n = 10 mesocosms per treatment) as follows: control (present-day temperature with present-day pCO2), ocean acidification (present-day temperature with + 350 μatm pCO2), ocean warming (+ 2 ℃ with present-day pCO2), and combined future ocean conditions (+ 2 °C with + 350 μatm pCO2). The elevated temperature and pCO2 levels are consistent with current commitments under the Paris Climate Agreement36. The ramets of M. capitata, P. compressa, and P. lobata were distributed among the 40 outdoor flow-through mesocosm tanks (70 L, 50 × 50 × 30 cm) at HIMB such that one ramet per genet was present within each of the four treatment conditions. Corals were maintained under experimental conditions for 22 months from 20 February 2016 to 13 December 2017 for a total of 662 days (Fig. S1). Representative photos of the mesocosm tanks for each treatment at the end of the experimental period are shown in Fig. 2. Salinity, temperature, pCO2, and pH were measured at mid-day in each mesocosm once weekly and treatment average weekly values (± 1SD) were plotted (Fig. 3). This is a long-term experiment as defined by McLachlan et al.37 and Grottoli et al.38 and the longest dual stress (i.e., combined ocean warming and acidification) experiment on corals to date (Table S3).
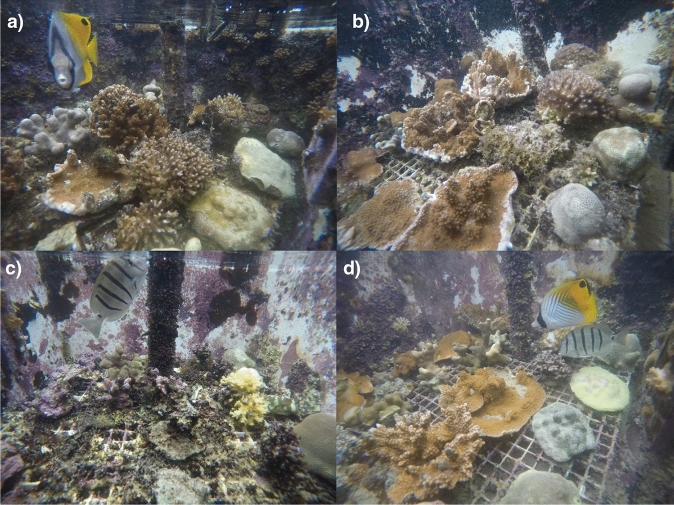
Figure 2.
Representative photos of the mesocosms after twenty-two months of exposure to: (a) control, (b) ocean acidification, (c) ocean warming, and (d) future ocean treatment conditions.
Figure 3.
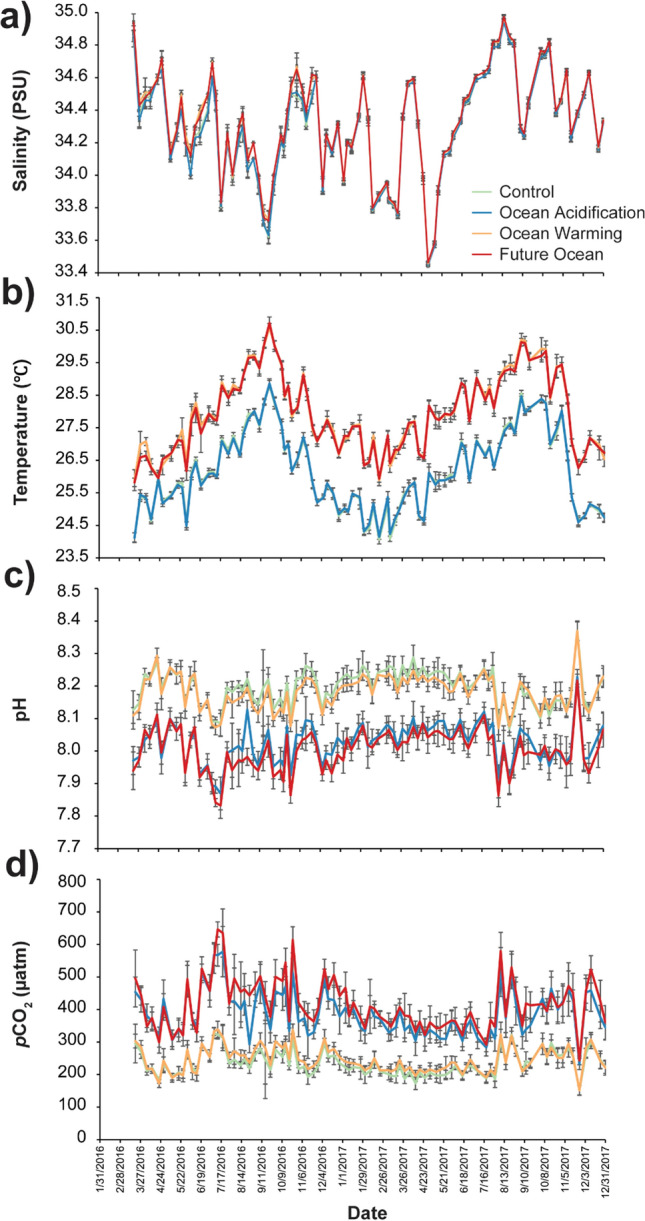
Weekly mean ± 1 SD mid-day (12:00) (a) salinity, (b) temperature, (c) pH, and (d) pCO2 in each experimental treatment: control (green), ocean acidification (blue), ocean warming (orange) and combined future ocean (red) throughout the 22-month experimental period. Dates provided as MM/DD/YYYY.
Coral fragments were photographed for surface area and ramet whiteness analysis, and buoyant weighed for growth rate on the weeks of 20 March 2016 and 27 November 2017 corresponding to one month after the target temperature and pH conditions were reached and the end of the experimental period, respectively (Fig. S1). Observed growth rates replicated near maximal growth rates observed in these species on the reef39, indicating that the mesocosms closely replicated reef conditions needed for optimal growth. Thus, findings in this study are likely to reflect responses expected under reef conditions. During the last 20 days of the experimental period (23 November–13 December 2017) the following live physiological measurements were conducted on all surviving coral ramets: photosynthesis, respiration, total organic carbon (TOC) flux, and maximum Artemia feeding capacity (Fig. S1). Equations associated with live measurements can be found in Table S4. Then, all surviving coral fragments were sacrificed by freezing at − 20 ℃. Samples were transported on dry ice to the Ohio State University (OH, USA) where they were stored at − 80 ℃ awaiting further analyses of biomass, lipids, proteins, Symbiodiniaceae density, and surface area according to methods published in protocols.io40–44. At least 10% of all live and post-sacrifice physiological measurements were made in duplicate. Additional details of the laboratory analysis methods are provided in the Supplemental Text.
In addition, the carbon budget of each coral ramet was calculated to determine the proportionate contribution of photosynthesis and heterotrophy to total metabolic demand (i.e., respiration). Photosynthesis and total respiration rates were used to calculate the percent Contribution of Zooxanthellae (i.e., Symbiodiniaceae) to Animal Respiration (CZAR)45, while total respiration and nighttime TOC flux rates were used to calculate the percent Contribution of Heterotrophy from TOC to Animal Respiration (CHARTOC)46. Artemia feeding capacity was not used to calculate CHARzoop as the Artemia concentrations were not representative of reef zooplankton densities or mesocosm zooplankton densities. The Contribution of the Total acquired fixed carbon relative to Animal Respiration (CTAR)27 was calculated as the sum of CZAR and CHARTOC. However, we acknowledge that this is likely an underestimate of CTAR as it does not account for heterotrophic carbon derived from zooplankton nor any potential gains or losses in CHARTOC that may have occurred during the day. Equations associated with carbon budget parameters can be found in Table S4.
Data analysis
To test for the effects of species temperature, and pCO2 on survivorship, survivorship data were analyzed using a generalized linear model with a binomial error distribution. To identify the physiological mechanisms underlying the ability of survivors of each species to cope (or not cope) with future ocean conditions, multivariate statistical analyses were performed. Ten physiological traits were standardized prior to the construction of Euclidean distance resemblance matrices. Data were visualized using non-parametric multidimensional scaling (NMDS) plots for each species. The effects of temperature and pCO2 (two-way analysis), and treatment (one-way analysis) on coral multivariate physiological profiles were investigated using permutational multivariate analysis of variance (PERMANOVA) and similarities percentage analysis (SIMPER). Univariate traits were analyzed using analysis of variance (ANOVA) and Tukey post-hoc tests. Additional details of the statistical analyses and software used are provided in the Supplemental Text.
Results and discussion
Which corals will survive chronic future ocean conditions?
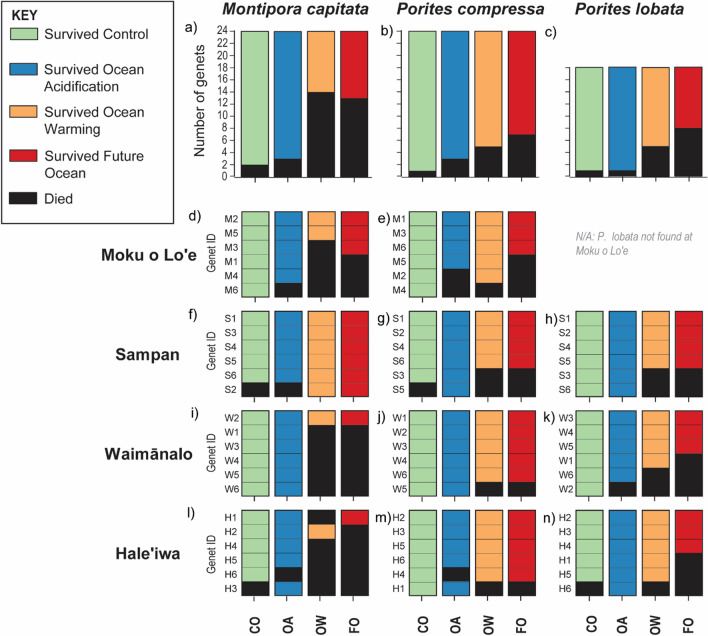
Coral survivorship was influenced by temperature treatment and coral species (Table S5). Overall, 61% of corals exposed to elevated temperature survived, whereas 92% survived under ambient temperature conditions (Fig. 4). Across treatments, Montipora capitata had significantly lower survivorship relative to Porites compressa (67% and 83% respectively, Fig. 4). Under the combined future ocean treatment, 46% of M. capitata, 71% of P. compressa, and 56% of P. lobata genets survived (Fig. 4). This indicates that in the future, under prolonged exposure to elevated temperature and pCO2, reefs will suffer a dramatic decline in coral cover and loss of genotypic diversity. However, between 46–71% of genets of three of the most common species in Hawaiʻi were able to survive (Fig. 4a–c), and no extirpation (100% mortality of all genets) was observed for any species from any site (Fig. 4 d–n). However, survival alone is not sufficient to evaluate the health and longevity of corals under combined future ocean conditions. For example, if corals are unable to calcify at sufficient rates, then reefs will not be able to keep up with sea-level rise or provide habitat and structure necessary for ecosystem function47. Likewise, if corals are unable to reproduce, then their fitness will be compromised and they will not contribute to future generations (i.e., so called “zombie” corals48). Finally, if corals are not able to meet metabolic demands through photosynthesis and/or heterotrophy, then they will eventually deplete energy reserves and die. Evaluating how surviving coral holobionts physiologically cope with future ocean conditions can reveal the longer-term prognosis for their health and persistence, and can be used to improve the accuracy of future projections of coral bleaching49.
Figure 4.
Survivorship of corals following 22 months exposure under control (CO, green), ocean acidification (OA, blue), ocean warming (OW, orange), or combined future ocean (FO, red) conditions for (a) M. capitata across sites, (b) P. compressa across site, (c) P. lobata across sites. Survivorship of the individual genets of each species collected from (d–e) Moku o Loʻe, (f–h) Sampan, (i–k) Waimānalo, and (l–n) Haleʻiwa.
How well do survivors cope with future ocean conditions?
The physiological profiles of Montipora capitata survivors were primarily influenced by temperature, whereas the interaction between temperature and pCO2 was found to be significant for both species of Porites (Table S6). The physiological profiles of the surviving corals under combined future ocean conditions significantly differed from the controls of Montipora capitata and Porites compressa (Fig. 5a, b, Table S7a, b), but not Porites lobata (Fig. 5c, Table S7c).
Figure 5.
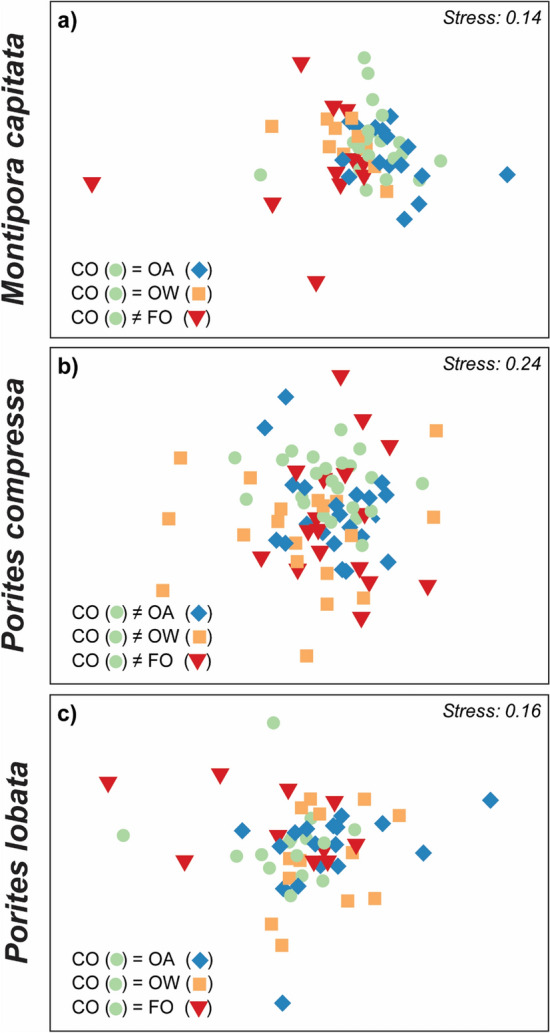
Nonparametric multidimensional scaling plots (NMDS) of coral physiological profiles for (a) Montipora capitata, (b) Porites compressa, and (c) Porites lobata. Data colors correspond to treatments: controls (CO, green circles), ocean acidification (OA, blue diamonds), ocean warming (OW, orange squares), or combined future ocean (FO, red triangles) treatments. Summary of pairwise PERMANOVA tests between CO and each of the treatments is shown in the bottom left of each panel. Additional statistical details in Table S7.
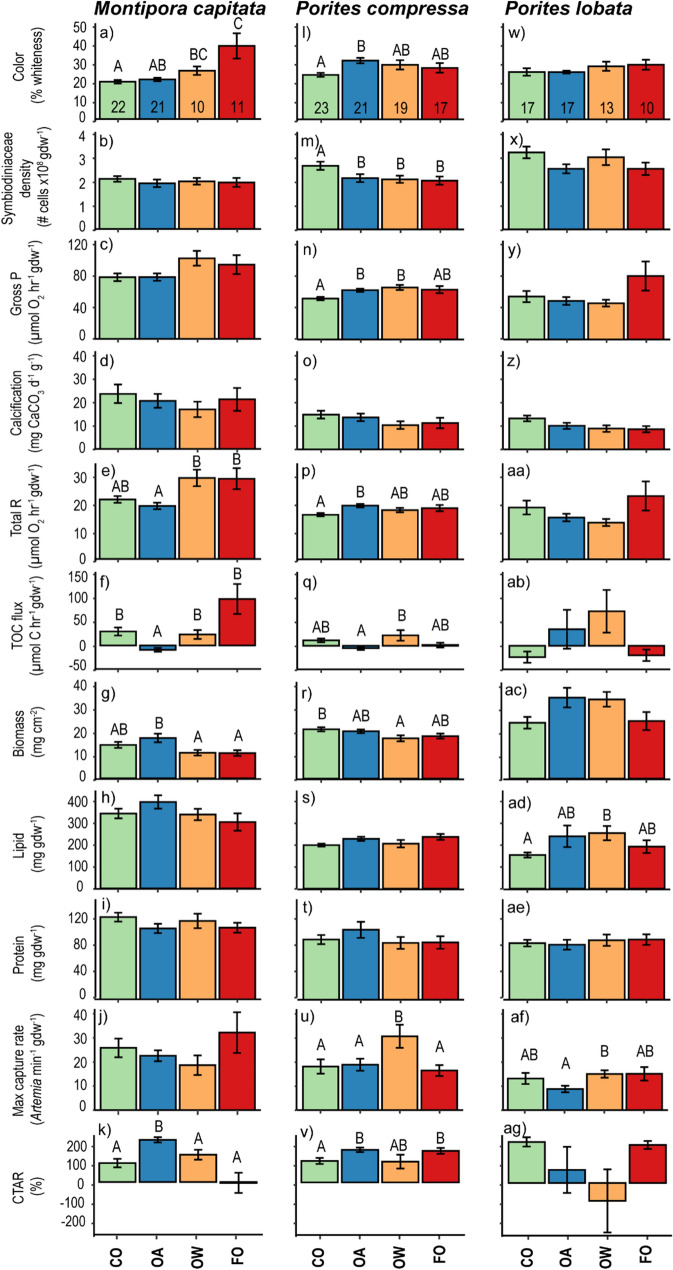
For M. capitata, the dissimilarity between combined future ocean and control physiological profiles was driven primarily by color, TOC flux, and maximum feeding capacity (Table S7a), of which the first two were higher under combined future ocean conditions compared to control conditions (Fig. 6a, f, j). The 20% increase in whiteness in M. capitata was due to degraded chlorophyll pigments while maintaining Symbiodiniaceae cell density (Fig. 6a, b)—a well-documented mechanism of bleaching for this species50. Though photosynthesis and calcification rates were unaffected by treatment (Fig. 6c, d), respiration rates increased by 35% under the ocean warming and future ocean conditions suggesting a metabolic cost to exposure to elevated temperature (Fig. 6e). Corals can supplement their carbon budget by increasing heterotrophy and/or catabolizing their tissues and energy reserves (e.g.,9,24,28,29). However, we found that M. capitata experienced an average 236% increase in TOC fluxes under combined future ocean conditions compared with controls (Fig. 6f), resulting in dramatic fixed carbon losses and decreases in CTAR (Fig. 6k) that fell to an average of -4%—well below the necessary 100% level—all while maintaining lipids, protein, and biomass (Fig. 6g, h, i). This is clearly unsustainable. These patterns suggest that the inability to fully meet metabolic demand under combined future ocean conditions may explain the higher mortality rates observed in M. capitata relative to P. compressa (Table S5). However, it is important to consider that bleached M. capitata are capable of substantially upregulating zooplankton heterotrophy to meet > 100% of their metabolic demand on the reef where demersal zooplankton concentrations are abundant28,51. In this study, M. capitata under future ocean conditions had the highest maximum feeding capacity measured (Fig. 4j), but zooplankton were not provided to the corals in the evening when corals typically have their polyps extended and feed52. Hence, corals like M. capitata which rely on heterotrophic food sources to survive and recover from bleaching will likely perform better in situ than observed here and may even have higher survivorship on reefs with higher natural zooplankton concentrations.
Figure 6.
Mean (±1SE) of physiological traits in (a-k) Montipora capitata, (l-v) Porites compressa, and (w-ag) Porites lobata following 22-months exposure to control (CO, green), ocean acidification (OA, blue), ocean warming (OW, orange), or combined future ocean (FO, red) experimental treatment conditions. Uppercase letters above bars indicate the results of post-hoc tests when ANOVA was significant, whereby averages sharing letters did not significantly differ from each other. Sample sizes for each variable are shown within each bar in the top row and is the same for all panels below. Statistical details in Table S8. TOC = Total Organic Carbon Flux. CTAR = Contribution of the Total acquired fixed carbon relative to Animal Respiration.
For Porites compressa, photosynthesis, respiration, and Symbiodiniaceae density were the main drivers of separation between the holobiont physiological profiles of control and the surviving future ocean treatment group (Table S7b). Symbiodiniaceae density was 23% lower in the combined future ocean treatment than in the controls (Fig. 6m). At the same time, the percent whiteness of P. compressa did not differ between the controls and combined future ocean treatment ramets (Fig. 6l), suggesting an increase in chlorophyll concentration per Symbiodiniaceae cell. This photo-acclimatory response has been previously observed in P. compressa50,51 and likely allowed this species to maintain the fixed carbon acquired through photosynthesis relative to the control group (Fig. 6n, v). Though photosynthesis, respiration, and TOC fluxes were not significantly different between the future ocean and control corals (Fig. 6n, p, q), the net effect of the future ocean conditions on the sum of all carbon budget variables resulted in a significant increase in CTAR values from 110% in the controls to 162% in the future ocean treatment survivors (Fig. 6v). Thus, P. compressa met > 100% of their metabolic energy requirements under combined future ocean conditions allowing this species to maintain energy reserves and calcification rates (Fig. 6o, r–t,), and these responses likely contributed to the low mortality rates observed in this species (Fig. 4b). Maximum Artemia capture rate did not change under the future ocean treatment, but increased 69% in the ocean warming treatment relative to the controls (Fig. 6u).
Unlike the other two species, P. lobata survivors under future ocean conditions did not significantly differ in their physiological profile relative to the controls (Fig. 5c, Table S7c) and no differences were found between the control and combined future ocean treatment ramets for any of the ten phenotypic traits measured (Fig. 6w–ag). Thus, the capacity for survivors of this species to cope with prolonged exposure to elevated temperature and pCO2 cannot be explained by any of the phenotypic traits measured here. Interestingly, the composition of the microbiome of P. lobata does differ between the control and combined future ocean treatment survivors53 suggesting that the microbiome may be a key element in the survival and coping mechanism of this species. We suggest that changes in physiological profile which occur in response to the single stress of either increased pCO2 or elevated temperature are counteracted when combined, and thus help drive resilience among survivors of combined future ocean conditions. This is especially apparent with CTAR, where corals under either ocean acidification or ocean warming failed to meet 100% of metabolic demand but exceeded 200% of metabolic demand under combined future ocean conditions and maintained calcification and energy reserves (Fig. 6ag).
Implications for future reefs
This is the longest ocean warming and acidification mesocosm experiment conducted on corals to date, and the only study to assess detailed holobiont physiological profiles following chronic, multiannual exposure to elevated temperature and pCO2 than include both diurnal and seasonal variability (Table S2). Our study sampled corals across a range of sites with differing environmental conditions, to help ensure that a representative sample of their phenotypic and genotypic diversity was included (Table S2). Therefore, this study provides insight into the capacity for corals to survive and identifies physiological traits that underly the capacity to cope with the future environmental change. We show that despite some mortality, at least two-thirds of P. compressa and P. lobata genets can survive and cope with future ocean conditions consistent with current commitments under the Paris Climate Agreement. While many survivors of M. capitata struggled to meet metabolic demand and cope, the lack of zooplankton supplementation may have unintentionally selected against this heterotrophically plastic species28,51 and unrealistically reduced survivorship. Nevertheless, we have shown that survivors of these three coral species are able to maintain calcification rates and tissue biomass, and maintain or increase lipid and protein energy reserves. In addition, both Porites coral species maintain or increase fixed carbon acquisition to meet or exceed their daily metabolic requirements. Thus, our finding provide hope that significant biological diversity within Porites, and several M. capitata genotypes, will persist this century provided atmospheric carbon dioxide levels are controlled within the commitments of the Paris Climate Agreement. Since Porites corals are ubiquitous throughout the world’s oceans, and P. lobata is a major reef builder in the Pacific, the resilience and persistence of Porites corals provides hope that some reef ecosystem function could be maintained globally.
Ethics approval
Samples were collected under State of Hawaiʻi Department of Land & Natural Resources Division of Aquatic Resources Special Activity Permit (SAP) #2015-48.
Supplementary Information
Acknowledgements
The authors thank J Altschuler, J Armstrong, A Arribas, L Bailey, K Bahr, E Barba, C Criswell, S Dixon, K Dobson, K Giesy, F Hawkes, H Hayes, A Huber, P Jokiel, C Juracka, E Kline, S Lannon, M Locatis, A Moore, M Moran, C Mortemore, L Mullins, B Nainiger, E Nguyen, Y Noggle, E O’Flynn, M Otto, K Rockwell, K Rodgers, K Ryan, E Sambuco, L Shizuru, A Smith, K Snyder, S Solomon, T Tran, J Walters, and A Wertz for assistance with field, laboratory, and computer analyses. Thank you to Dr. C Wall for statistical assistance and advice. Thank you to Dr. L Krissek and Dr. L Chapron for providing feedback and review on the manuscript draft.
Author contributions
A.G.G., C.P.J., and R.J.T. conceived of the study, wrote proposals, and secured funding. A.G.G. and R.J.T. supervised the study. C.P.J. collected samples, conducted and maintained the overall mesocosm experiment, and performed the calcification measurements; S.L. analyzed TOC water samples; R.H.M. and J.T.P. performed the other laboratory analyses; N.W. and A.M.G. assisted with method development and facilitated use of laboratory equipment; R.H.M. conducted the data analysis and wrote the manuscript. A.G.G. contributed substantially to data interpretation and manuscript revision. All authors contributed critically to drafts and gave final approval for publication.
Funding
AGG obtained major funding for this research from the National Science Foundation OCE Division of Ocean Sciences (award number: 1459536). Additional support to AGG came from the HW Hoover Foundation and the National Science Foundation OCE Division of Ocean Sciences (award number 1838667) and to CPJ and RJT from UH Sea Grant (award number: 2180), the National Science Foundation Ocean Acidification Program (award number: OA-1416889), and NSF OCE Division of Ocean Sciences (award number: 1514861). RHM obtained funding for some sample analyses from Sigma Xi—The Scientific Honor Society (National and Ohio State Chapter Awards).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The raw data analyzed for this study are deposited at BCO-DMO https://www.bco-dmo.org/project/546273.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rowan H. McLachlan, Email: mclachlan.8@osu.edu
Andréa G. Grottoli, Email: grottoli.1@osu.edu
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06896-z.
References
- 1.Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, Van De Leemput IA, Lough JM, Morrison TH, Palumbi SR, Van Nes EH, Scheffer M. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 2.Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs JPA, Hoogenboom MO, Kennedy EV, Kuo CY, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom MO, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett MS, Schoepf V, Torda G, Wilson SK. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science (80- ). 2018;359:80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- 4.Eakin CM, Sweatman HPA, Brainard RE. The 2014–2017 global-scale coral bleaching event: Insights and impacts. Coral Reefs. 2019;38:539–545. [Google Scholar]
- 5.Glynn Coral reef bleaching: Facts, hypotheses and implications. Glob. Chang. Biol. 1996;2:495–509. [Google Scholar]
- 6.Brown BE. Coral bleaching: Causes and consequences. Coral Reefs. 1997;16:129–138. [Google Scholar]
- 7.Maynard JA, Van Hooidonk R, Eakin CM, Puotinen M, Garren M, Williams G, Heron SF, Lamb J, Weil E, Willis BL, Harvell CD. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat. Clim. Chang. 2015;5:688–694. [Google Scholar]
- 8.Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G. Global warming transforms coral reef assemblages. Nature. 2018;556:492–496. doi: 10.1038/s41586-018-0041-2. [DOI] [PubMed] [Google Scholar]
- 9.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Yuan XC, Cai WJ, Zhang CL, Li X, Liu S. Positive and negative responses of coral calcification to elevated pCO2: Case studies of two coral species and the implications of their responses. Mar. Ecol. Prog. Ser. 2014;502:145–156. [Google Scholar]
- 11.Hoadley KD, Pettay DT, Grottoli AG, Cai WJ, Melman TF, Schoepf V, Hu X, Li Q, Xu H, Wang Y, Matsui Y, Baumann JH, Warner ME. Physiological response to elevated temperature and pCO2 varies across four Pacific coral species: Understanding the unique host + symbiont response. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep18371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoepf V, Grottoli AG, Warner ME, Cai WJ, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H, Wang Y, Matsui Y, Baumann JH. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS One. 2013;8:e75049. doi: 10.1371/journal.pone.0075049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IPCC. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, (eds. Pörtner, H.-O. et al.) 1–36 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2019).
- 14.Bahr KD, Jokiel PL, Rodgers KS. Relative sensitivity of five Hawaiian coral species to high temperature under high-pCO2 conditions. Coral Reefs. 2016;35:729–738. [Google Scholar]
- 15.Dove SG, Brown KT, Van Den Heuvel A, Chai A, Hoegh-Guldberg O. Ocean warming and acidification uncouple calcification from calcifier biomass which accelerates coral reef decline. Commun. Earth Environ. 2020;1:1–9. [Google Scholar]
- 16.Chow MH, Tsang RHL, Lam EKY, Ang PO. Quantifying the degree of coral bleaching using digital photographic technique. J. Exp. Mar. Bio. Ecol. 2016;479:60–68. [Google Scholar]
- 17.Amid C, Olstedt M, Gunnarsson JS, Le Lan H, Tran Thi Minh H, Van den Brink PJ, Hellström M, Tedengren M. Additive effects of the herbicide glyphosate and elevated temperature on the branched coral Acropora formosa in Nha Trang, Vietnam. Environ. Sci. Pollut. Res. 2018;25:13360–13372. doi: 10.1007/s11356-016-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony KRN, Connolly SR, Willis BL. Comparative analysis of energy allocation to tissue and skeletal growth in corals. Limnol. Oceanogr. 2002;47:1417–1429. [Google Scholar]
- 19.Edmunds PJ, Davies PS. An energy budget for Porites porites (Scleractinia) Mar. Biol. 1986;92:339–347. [Google Scholar]
- 20.Stimson JS. Location, quantity and rate of change in quantity of lipids in tissue of Hawaiian hermatypic corals. Bull. Mar. Sci. 1987;41:889–904. [Google Scholar]
- 21.Harland AD, Navarro JC, Spencer Davies P, Fixter LM. Lipids of some Caribbean and Red Sea corals: Total lipid, wax esters, triglycerides and fatty acids. Mar. Biol. 1993;117:113–117. [Google Scholar]
- 22.Grottoli AG, Tchernov D, Winters G. Physiological and biogeochemical responses of super-corals to thermal stress from the northern gulf of Aqaba, Red Sea. Front. Mar. Sci. 2017;4:1–12. [Google Scholar]
- 23.Rodrigues LJ, Grottoli AG. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 2007;52:1874–1882. [Google Scholar]
- 24.Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R. Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Funct. Ecol. 2009;23:539–550. [Google Scholar]
- 25.Baumann JH, Grottoli AG, Hughes AD, Matsui Y. Photoautotrophic and heterotrophic carbon in bleached and non-bleached coral lipid acquisition and storage. J. Exp. Mar. Bio. Ecol. 2014;461:469–478. [Google Scholar]
- 26.Hughes AD, Grottoli AG. Heterotrophic compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0081172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grottoli AG, Warner ME, Levas SJ, Aschaffenburg MD, Schoepf V, McGinley M, Baumann JH, Matsui Y. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Chang. Biol. 2014;20:3823–3833. doi: 10.1111/gcb.12658. [DOI] [PubMed] [Google Scholar]
- 28.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 29.Levas SJ, Grottoli AG, Schoepf V, Aschaffenburg MD, Baumann JH, Bauer JE, Warner ME. Can heterotrophic uptake of dissolved organic carbon and zooplankton mitigate carbon budget deficits in annually bleached corals? Coral Reefs. 2016;35:495–506. [Google Scholar]
- 30.Jury CP, Delano MN, Toonen RJ. High heritability of coral calcification rates and evolutionary potential under ocean acidification. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-56313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jury CP, Toonen RJ. Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proc. R. Soc. B Biol. Sci. 2019;286:20190614. doi: 10.1098/rspb.2019.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Concepcion GT, Polato NR, Baums IB, Toonen RJ. Development of microsatellite markers from four Hawaiian corals: Acropora cytherea, Fungia scutaria, Montipora capitata and Porites lobata. Conserv. Genet. Resour. 2010;2:11–15. [Google Scholar]
- 33.Gorospe KD, Karl SA. Genetic relatedness does not retain spatial pattern across multiple spatial scales: Dispersal and colonization in the coral, Pocillopora damicornis. Mol. Ecol. 2013;22:3721–3736. doi: 10.1111/mec.12335. [DOI] [PubMed] [Google Scholar]
- 34.Wall CB, Ritson-Williams R, Popp BN, Gates RD. Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery. Limnol. Oceanogr. 2019;64:2011–2028. doi: 10.1002/lno.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahr KD, Tran T, Jury CP, Toonen RJ. Abundance, size, and survival of recruits of the reef coral Pocillopora acuta under ocean warming and acidification. PLoS ONE. 2020;15:1–13. doi: 10.1371/journal.pone.0228168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogelj J, Den Elzen M, Höhne N, Fransen T, Fekete H, Winkler H, Schaeffer R, Sha F, Riahi K, Meinshausen M. Paris agreement climate proposals need a boost to keep warming well below 2 °C. Nature. 2016;534:631–639. doi: 10.1038/nature18307. [DOI] [PubMed] [Google Scholar]
- 37.McLachlan RH, Price JT, Solomon SL, Grottoli AG. Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs. 2020;39:885–902. [Google Scholar]
- 38.Grottoli AG, et al. Increasing comparability among coral bleaching experiments. Ecol. Appl. 2021;31:e02262. doi: 10.1002/eap.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grottoli AG. Variability of stable isotopes and maximum linear extension in reef-coral skeletons at Kaneohe Bay, Hawaii. Mar. Biol. 1999;135:437–449. [Google Scholar]
- 40.McLachlan, R. H., Dobson, K. L., Grottoli, A. G. Quantification of Total Biomass in Ground Coral Samples. Protocols.io (2020). 10.17504/protocols.io.bdyai7se.
- 41.McLachlan, R. H., Muñoz-Garcia, A., Grottoli, A. G. Extraction of Total Soluble Lipid from Ground Coral Samples. Protocols.io (2020). 10.17504/protocols.io.bc4qiyvw.
- 42.McLachlan, R. H., Price, J. T., Dobson, K. L., Weisleder, N. & Grottoli, A. G. Microplate Assay for Quantification of Soluble Protein in Ground Coral Samples. Protocols.io (2020). 10.17504/protocols.io.bdc8i2zw.
- 43.McLachlan, R. H., Juracka, C. & Grottoli, A. G. Symbiodiniaceae Enumeration in Ground Coral Samples Using Countess™ II FL Automated Cell Counter. Protocols.io (2020). 10.17504/protocols.io.bdc5i2y6.
- 44.McLachlan, R. H. & Grottoli, A. G. Geometric Method for Estimating Coral Surface Area Using Image Analysis. Protocols.io10.17504/protocols.io.bdyai7se(2021).
- 45.Muscatine L, McCloskey LR, Marian RE. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 1981;26:601–611. [Google Scholar]
- 46.Levas SJ, Grottoli AG, Warner ME, Cai WJ, Bauer JE, Schoepf V, Baumann JH, Matsui Y, Gearing C, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H, Wang Y. Organic carbon fluxes mediated by corals at elevated pCO2 and temperature. Mar. Ecol. Prog. Ser. 2015;519:153–164. [Google Scholar]
- 47.Perry CT, Alvarez-Filip L, Graham NAJ, Mumby PJ, Wilson SK, Kench PS, Manzello DP, Morgan KM, Slangen ABA, Thomson DP, Januchowski-Hartley F, Smithers SG, Steneck RS, Carlton R, Edinger EN, Enochs IC, Estrada-Saldívar N, Haywood MDE, Kolodziej G, Murphy GN, Pérez-Cervantes E, Suchley A, Valentino L, Boenish R, Wilson M, MacDonald C. Loss of coral reef growth capacity to track future increases in sea level. Nature. 2018;558:396–400. doi: 10.1038/s41586-018-0194-z. [DOI] [PubMed] [Google Scholar]
- 48.Woodley, C. M., Burnett, A. & Downs, C. A. Epidemiological Assessment of Reproductive Condition of ESA Priority Coral (2013).
- 49.Logan CA, Dunne JP, Eakin CM, Donner SD. Incorporating adaptive responses into future projections of coral bleaching. Glob. Chang. Biol. 2014;20:125–139. doi: 10.1111/gcb.12390. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues LJ, Grottoli AG, Lesser MP. Long-term changes in the chlorophyll fluorescence of bleached and recovering corals from Hawaii. J. Exp. Biol. 2008;211:2502–2509. doi: 10.1242/jeb.012369. [DOI] [PubMed] [Google Scholar]
- 51.McLachlan Rowan H., Price James T., Muñoz-Garcia Agustí, Weisleder Noah L., Jury Christopher P., Toonen Robert J., Grottoli Andréa G. Environmental gradients drive physiological variation in Hawaiian corals. Coral Reefs. 2021;40(5):1505–1523. doi: 10.1007/s00338-021-02140-8. [DOI] [Google Scholar]
- 52.Houlbrèque F, Ferrier-Pagès C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009;84:1–17. doi: 10.1111/j.1469-185X.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- 53.J. T. Price, thesis, The Ohio State University (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The raw data analyzed for this study are deposited at BCO-DMO https://www.bco-dmo.org/project/546273.