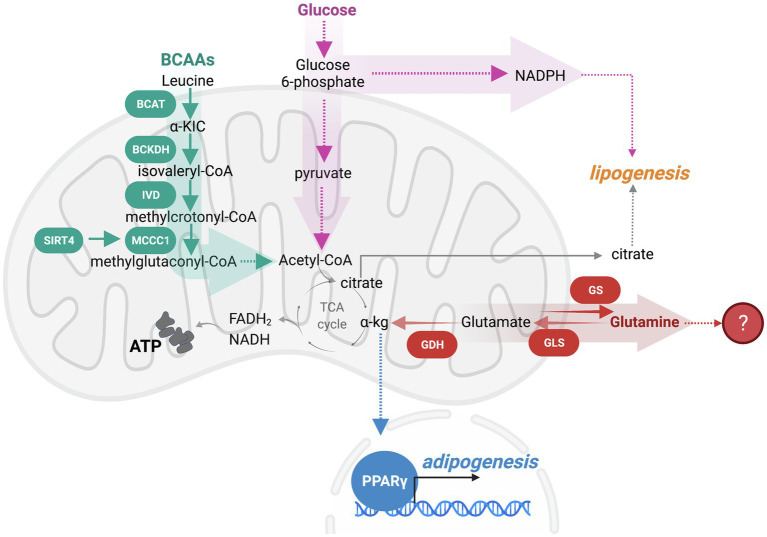
Figure 1.
Nutrient oxidation in the mitochondria modulates adipogenesis. Branched-chain amino acids (BCAAs) and glucose catabolism within the mitochondria produce ATP and metabolic intermediates that promote adipogenesis. As such, leucine catabolism is an early regulator of adipogenesis that is initiated by a BCAA transaminase (BCAT) generating α-ketoisocaproic acid (a-KIC). a-KIC is irreversibly oxidized by the BCAA dehydrogenase (BCKDH) complex forming isovaleryl-CoA. Isovaleryl-CoA dehydrogenase (IVD) converts isovaleryl-CoA to methylcrotonyl-CoA. Sirtuin SIRT4 induction of methylcrotonyl-CoA carboxylase (MCCC1) promotes the carboxylation of 3-methylcrotonyl-CoA to 3-methylglutaconyl-CoA, which can be further oxidized to produce acetyl-CoA. BCAA catabolism promotes the PPARγ-mediated transcriptional adipogenic program. While a portion of glucose is oxidized to generate acetyl-CoA, some glucose is diverted away from the mitochondrial oxidation to the pentose phosphate pathway (PPP) to support the production of nicotinamide adenine dinucleotide phosphate (NADPH), a required cofactor for lipogenesis. Glutaminolysis appears to oppose adipogenesis, although the mechanistic understanding of fuel switching that supports adipogenesis is limited.