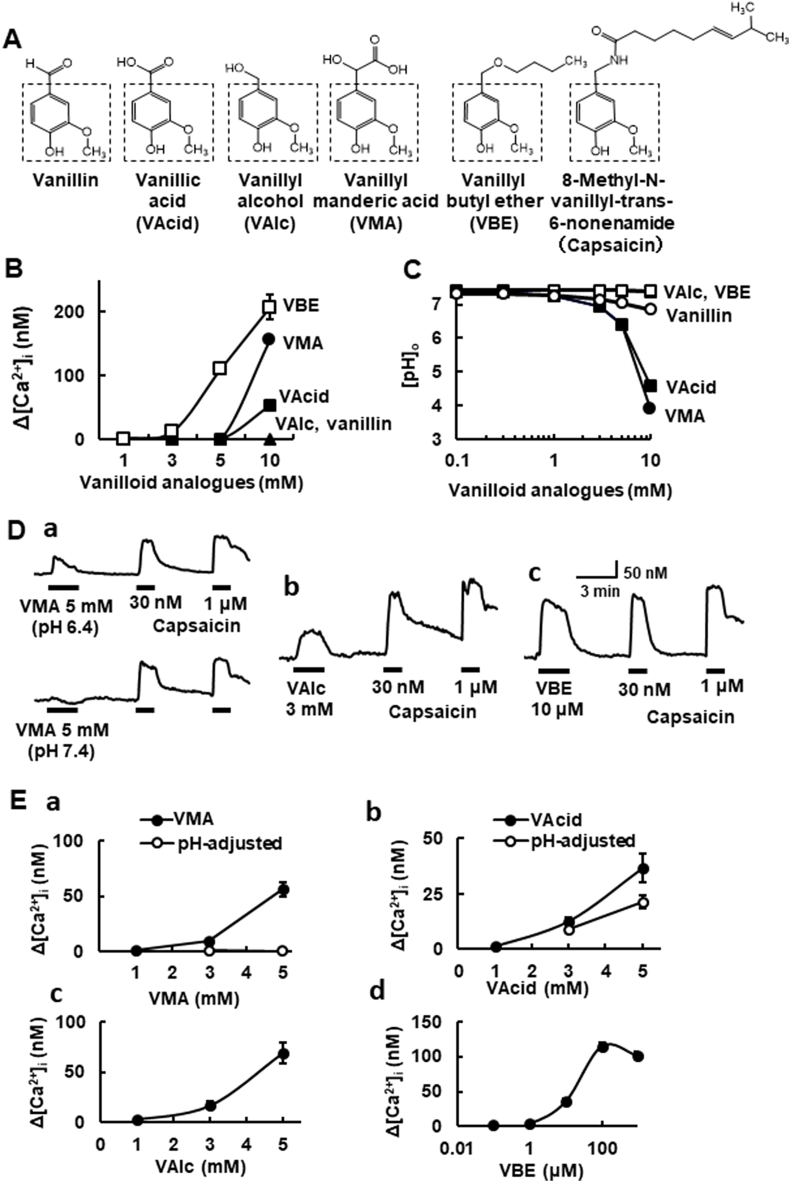
Fig. 1.
[Ca2+]i responses to vanilloid analogues in naïve HEK293 cells and those expressing mTRPV1. (A) Structures of vanilloid analogues used in the present study. The vanillyl skeleton is surrounded by a dashed square. (B) The concentration-[Ca2+]i response curves for the vanilloid analogues. Symbols with vertical lines show mean ± SEM (open squares: VBE; n = 20, closed circles: VMA; n = 100, closed squares: VAcid; n = 92). VAlc (n = 100) and vanillin (n = 100) did not change [Ca2+]i up to 10 mM (closed triangles). (C) The concentration-pH curves for the vanilloid analogue-dissolved solutions. Note that the solutions dissolved in VAcid and VMA at 10 mM are highly acidic. (D) Actual recordings of [Ca2+]i responses to the vanilloid analogues (a; VMA, b; VAlc, c; VBE) and capsaicin (30 nM, 1 μM). In VMA, upper (pH 6.4 solution) and lower (adjusted to pH 7.4). (E) The concentration-relation of four vanilloid analogues. (a) VMA (closed symbols; pH-unadjusted, open ones; pH 7.4-adjusted, n = 18–48), (b) VAcid (closed symbols; pH-unadjusted, open ones; pH 7.4-adjusted, n = 41–68), (c) VAlc (n = 8–47) and (d) VBE (n = 18–75). Symbols with vertical lines show mean ± SEM.