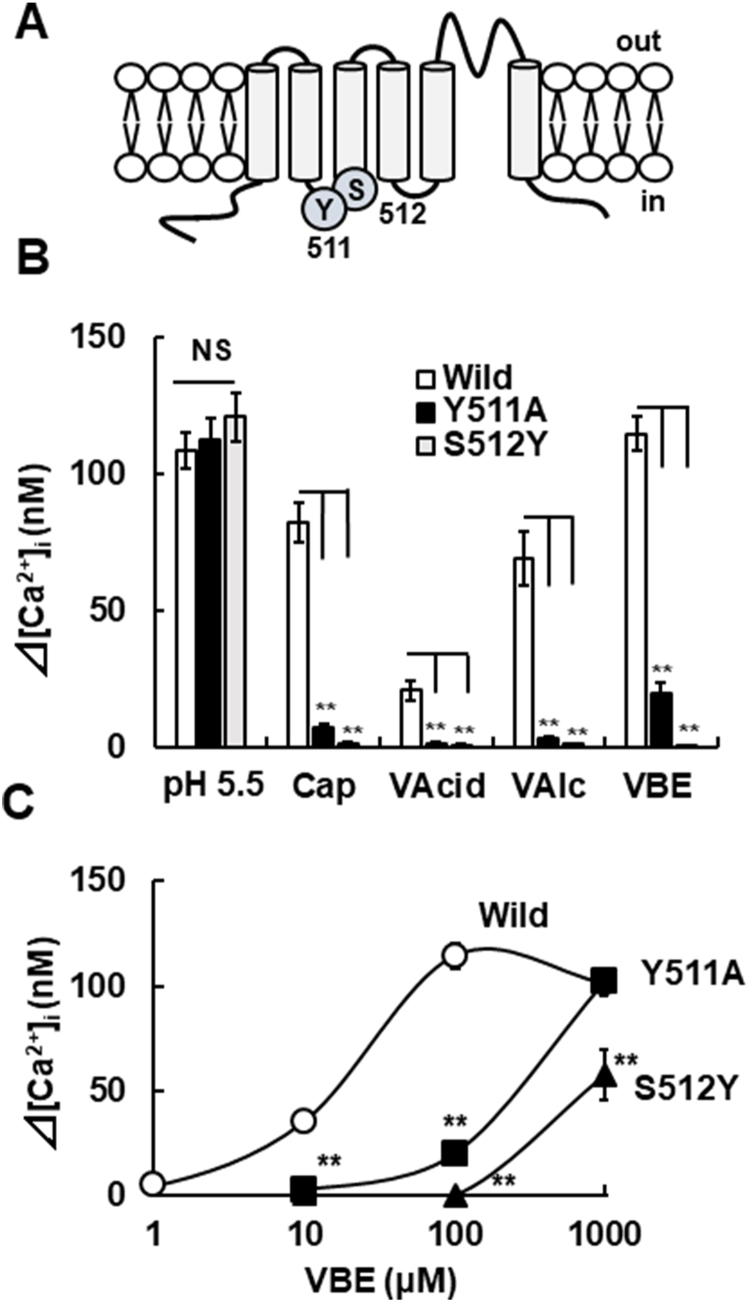
Fig. 2.
Interactions of vanilloid analogues to capsaicin-sensing sites for the channel activation. (A) A schematic of the mTRPV1 structure including the capsaicin-sensitive amino acid residues tyrosine 511 (Y511) and serine 512 (S512). (B) The amplitudes of [Ca2+]i increases induced by pH 5.5, capsaicin (300 nM), VAcid (5 mM)-adjusted pH 7.4, VAlc (5 mM) and VBE (100 nM) in wild (open columns), Y511A (closed columns) and S512Y (grey columns). Columns with vertical lines show mean ± SEM (open column: wild, pH 5.5; n = 71, cap; n = 31, VAcid; n = 24, VAlc; n = 26, VBE; n = 75, closed column: Y511A, pH 5.5; n = 22, cap; n = 22, VAcid; n = 19, VAlc; n = 27, VBE; n = 28, and grey column: S512Y, pH 5.5; n = 30, cap; n = 30, VAcid; n = 35, VAlc; n = 47, VBE; n = 38). (C) The concentration-[Ca2+]i response curves for VBE [open circles; Wild mTRPV1-HEK, closed squares; mTRPV1 (Y511A)-HEK, closed triangles; mTRPV1 (S512A)-HEK]. Symbols with vertical lines show mean ± SEM (Wild, 10 μM; n = 40, 100 μM; n = 75, 1000 μM; n = 63, mTRPV1 (Y511A)-HEK, 10 μM, 100 μM; n = 28, 1000 μM; n = 29, mTRPV1 (S512Y) -HEK, 10 μM, 100 μM; n = 38, 1000 μM; n = 21). **, p < 0.01 vs. Wild (control) by one-way ANOVA with Tukey-Kramer test or Dunnett T3 test, NS, not significant.