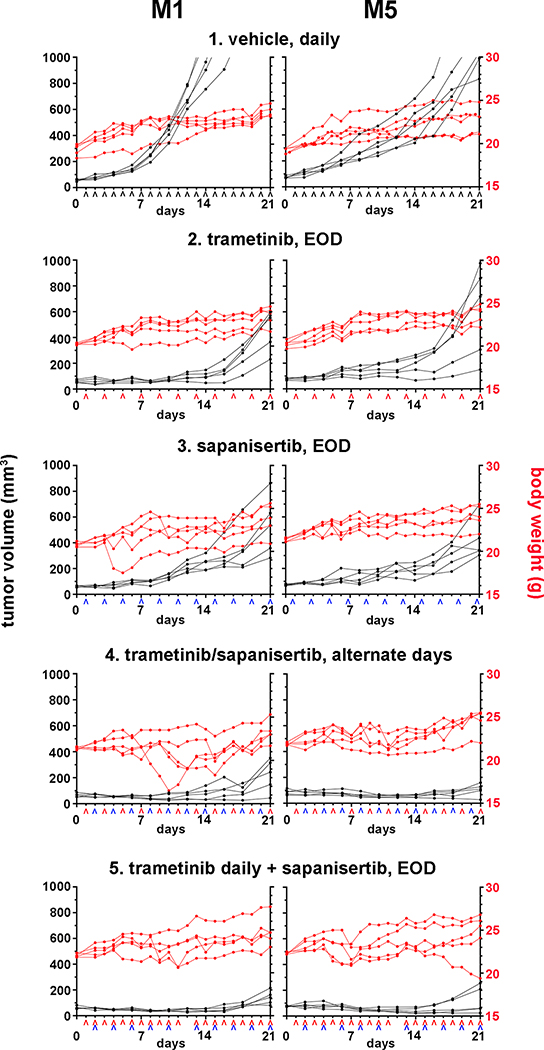
Figure 3.
Pre-clinical therapeutic efficacy and tolerability in a staggered repeat-dose treatment regimen. Nude mice bearing M1 or M5 canine MM subcutaneous xenografts were randomly divided into five treatment groups, 1) vehicle, daily, 2) trametinib, every other day (EOD), 3) sapanisertib, every other day, 4) trametinib and sapanisertib on alternate days, and 5) trametinib daily plus sapanisertib every other day, (n=5 mice per group). Trametinib (^ red caret) was given at 5.0 mg/Kg and sapanisertib (^ blue caret) at 2.0 mg/Kg; the treatment schedule is labeled along the x-axis. The body weights and tumor volumes were followed through the 21-day treatment duration.