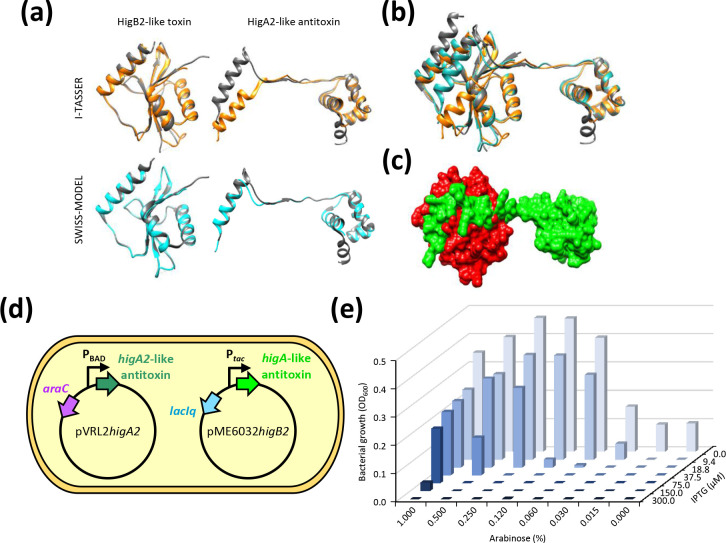
Fig. 7.
HigB2-like and HigA2-like components the TA system of p1ATCC19606. (a) Superimposition of the HigBA2-like TA complex on the Vibrio cholerae HigBA2 TA crystal structure (5JAA). The query structure is shown in grey, while the structural analogue is displayed in orange or cyan for I-TASSER- and SWISS-MODEL-based models, respectively. Only the first-ranked model predicted by I-TASSER and SWISS-MODEL for each query is shown. Torsion angles of amminoacid residues 26–30 of the I-TASSER-based model of the predicted HigA2-like antitoxin were modified to orient the α-helix involved in the interaction with HigB2-like toxin. (b) Superimposition of the predicted p1ATCC19606 TA complex models (I-TASSER, orange; SWISS-MODEL, cyan) over the crystal structure of HigB2-HigA2 (grey; 5JAA). (c) GRASP surface representation of the HigB2-like toxin (red)-HigA2-like antitoxin (green) complex based on the SWISS-MODEL predictions, displaying the interaction between the putative toxin and antitoxin proteins. The images shown in (a–c) were obtained using UCSF Chimaera. (d) Schematic illustration of HigB2-like toxin neutralization by the HigA2-like antitoxin. The arabinose-inducible expression of the higA2-like antitoxin gene provided in trans from pVRL2 allows the growth of E. coli DH5α expressing the IPTG-inducible higB2-like toxin gene from plasmid pME6032higB2. (e) Bacterial growth assessed after 24 h incubation at 37 °C in LB supplemented with the appropriate antibiotic concentration. To induce the expression of the higA2-like antitoxin gene from the arabinose-inducible PBAD promoter and of the higB2-like toxin gene from the IPTG-inducible P tac promoter, the medium was supplemented with the indicated arabinose and IPTG concentrations, respectively. OD600 values are representative of three independent experiments giving similar results.