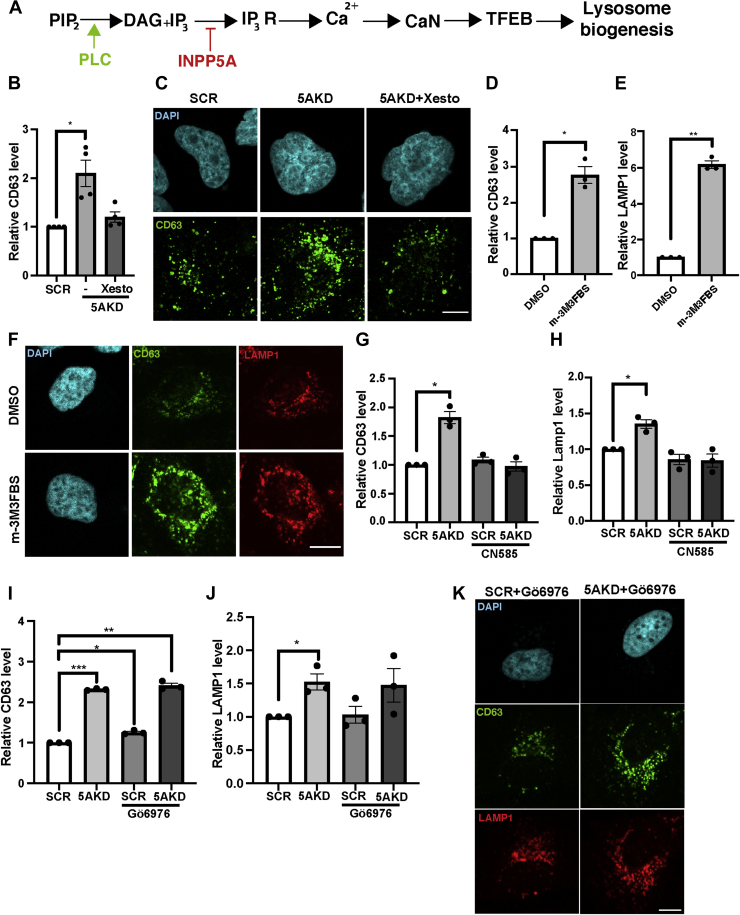
Figure 3.
IP3-induced calcium release increases cellular lysosome content downstream of receptor signaling via phospholipase C and Ca2+/calcineurin.A, schematic representation of phospholipase C (PLC)-mediated cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and IP3 downstream of receptor signaling. IP3 triggers Ca2+ release from the ER via IP3 receptors (IP3Rs), a pathway repressed by INPP5A-mediated IP3 hydrolysis. Ca2+ release activates calcineurin (CaN), which dephosphorylates and thereby activates TFEB to induce lysosomal gene expression. B, quantification of relative CD63 levels in representative data shown in C. Data for SCR-control siRNA-treated cells were set to 1. One-sample Student's t test followed by Benjamini–Hochberg correction. -/5AKD: p = 0.0267, t = 4.072, and df = 3. Xesto/5AKD: p = 0.1660, t = 1.822, and df = 3. C, representative confocal images of fixed HeLa cells treated with control (SCR) or INPP5A siRNA (5AKD) treated with DMSO (−) or with 1 μM xestospongin C (Xesto) for 18 h and stained for CD63 (green). Blue, DAPI-stained nuclei. The scale bar represents 10 μm. D and E, quantification of representative data shown in F. Data for SCR-control siRNA-treated cells were set to 1. One-sample Student's t test. CD63/m-3M3FBS: p = 0.0173, t = 7.506, and df = 2. LAMP1/m-3M3FBS: p = 0.0017, t = 24.14, and df = 2. F, representative confocal images of fixed HeLa cells treated with DMSO or with 1 μM m-3M3FBS for 24 h and stained with specific antibodies for CD63 (green) and LAMP1 (red). Blue, DAPI-stained nuclei. The scale bar represents 10 μm. Zoomed views of low magnification images shown in Fig. S5B are shown. G and H, quantification of relative levels of CD63 (G) and LAMP1 (H), in HeLa cells treated with DMSO or with 5 μM CN585 for 24 h. Data for SCR-control siRNA-treated cells were set to 1. One-sample Student's t test followed by Benjamini–Hochberg correction. CD63/5AKD: p = 0.0154, t = 7.968, and df = 2. CD63/SCR + CN585: p = 0.2318, t = 1.697, and df = 2. CD63/5AKD + CN585: p = 0.7773, t = 0.3231, and df = 2. LAMP1/5AKD: p = 0.0287, t = 5.774, and df = 2. LAMP1/SCR + CN585: p = 0.182, t = 2.011, and df = 2. LAMP1/5AKD + CN585: p = 0.2254, t = 1.732, and df = 2. I and J, quantification of relative levels of CD63 (I) and LAMP1 (J) in HeLa cells treated with control (SCR) or INPP5A siRNA (5AKD) and treated with DMSO or 100 nM CN585 for 24 h. Data for DMSO-treated SCR-control siRNA-treated cells were set to 1. One-sample Student’s t test followed by Benjamini–Hochberg correction. CD63/5AKD: p = 0.0001, t = 90.39, and df = 2. CD63/SCR + Gö6976: p = 0.0111, t = 9.407, and df = 2. CD63/5AKD + Gö6976: p = 0.0017, t = 24.32, and df = 2. LAMP1/5AKD: p = 0.0497, t = 4.317, and df = 2. LAMP1/SCR + Gö6976: p = 0.8347, t = 0.2369, and df = 2. LAMP1/5AKD + Gö6976: p = 0.2036, t = 1.863, and df = 2. K, representative confocal images of fixed HeLa cells treated with control (SCR) or INPP5A siRNA (5AKD) and with or without 100 nM Gö6976 (24 h) and stained with specific antibodies against CD63 (green) or LAMP1 (red). Blue, DAPI-stained nuclei. The scale bar represents 10 μm. Data represent n = 4 independent experiments for xestospongin C treatment, n = 3 for treatments with m-3M3FBS, CN585, and Gö6976. DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; ER, endoplasmic reticulum; INPP5A, inositol polyphosphate-5-phosphatase A; IP3, inositol triphosphate; LAMP1, lysosome-associated membrane protein 1; TFEB, transcription factor EB.