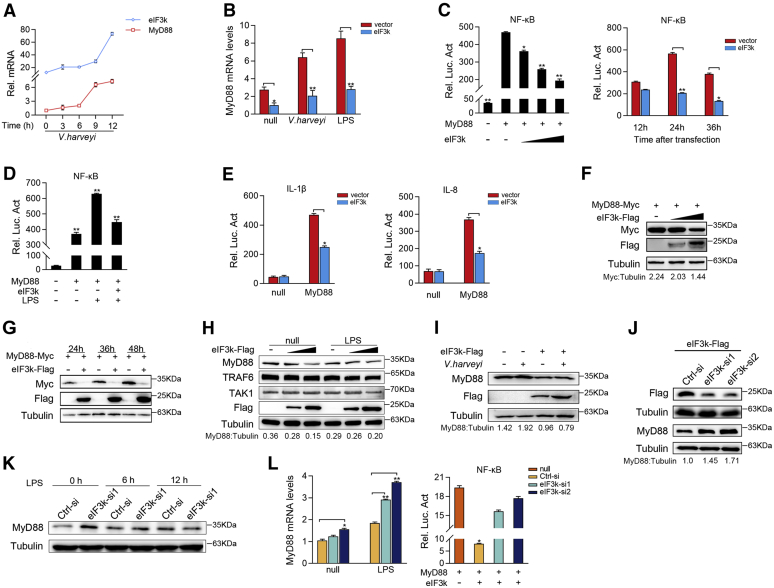
Figure 3.
eIF3k terminates NF-κB signaling by degrading MyD88.A, expression of eIF3k and MyD88 mRNAs in MIC was determined by qPCR after V. harveyi stimulation at for different times. B, eIF3k inhibited the transcription of endogenous MyD88. MIC cells were transfected with the indicated plasmids for 24 h, and then stimulated with V. harveyi and LPS for 6 h. eIF3k and MyD88 mRNAs were determined by qPCR. C–E, eIF3k inhibits myD88-induced NF-κB signaling. EPC cells were transfected with pRL-TK, luciferase reporter genes, together with eIF3k. Luciferase activity was measured and normalized to renilla luciferase activity. F, eIF3k inhibited MyD88 in a dose-dependent manner. EPC cells were co-transfected with MyD88 and eIF3k for 48 h, the expression of MyD88 was determined by immunoblot. G, eIF3k inhibited MyD88 in a schedule-dependent manner. EPC cells were co-transfected with MyD88 and eIF3k; cells were collected at different point for immunoblot. H, eIF3k specifically targets MyD88. MIC cells were transfected with eIF3k or control vector for 48 h. Endogenous proteins were determined by immunoblot. I, eIF3k inhibits V. harveyi-triggered MyD88. MIC cells were infected with V. harveyi for 6 h at post-transfected with eIF3k. Endogenous MyD88 was determined by immunoblot. J–L, knockdown of eIF3k slowed the degradation of endogenous MyD88. MIC cells were transfected with eIF3k-si and Ctrl-si (J), after LPS treatment for 6 h and 12 h (K), the expression of MyD88 was determined by immunoblot and qPCR (L). The data are shown as the mean ± SD of three independent experiments. (∗) p< 0.05, (∗∗) p< 0.01 versus the controls. eIF3k, eukaryotic translation initiation factor 3k; EPC, epithelioma papulosum cyprini; MIC, M. miiuy intestine cell.