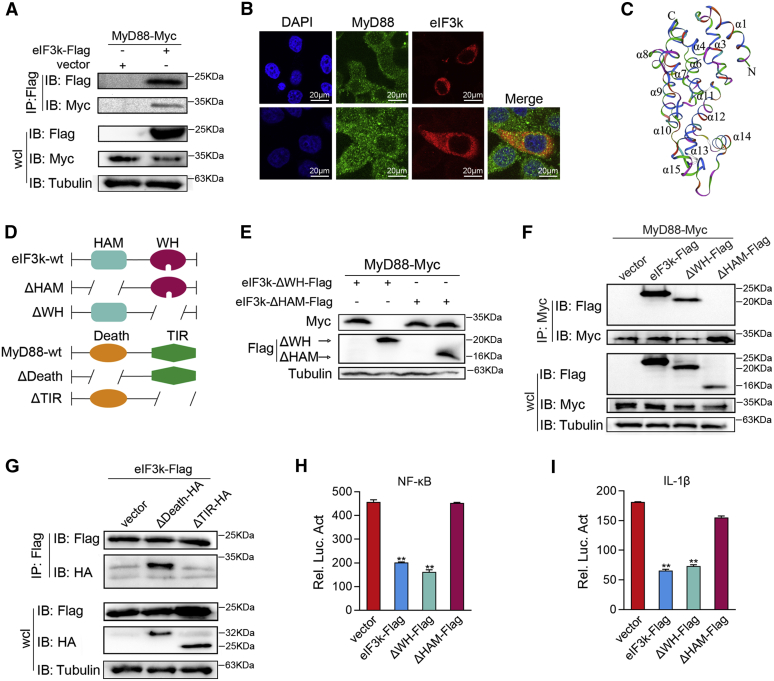
Figure 4.
Identification of eIF3k as a MyD88-interacting partner.A, eIF3k interacts with MyD88. HEK 293 cells were transfected with the indicated plasmids for 32 h. Co-IP was performed and subjected to immunoblot analysis. B, eIF3k colocalizes with MyD88 in cytoplasm. HeLa cells were transfected with the indicated plasmids for 30 h, then stained with FITC (green) or Cy3 (red). The nuclei were stained by DAPI (blue). Pictures were taken by FCFM. Scale bar, 20 μm; original magnification ×40. C, the overall of eIF3k was predicted by the Swiss model. D, the truncated mutants of MyD88 and eIF3k. E–G, MyD88 associates with eIF3k through its TIR and HAM domain. EPC cells (E) and HEK293 cells (F and G) were transfected with the indicated plasmids for immunoblot (E) and mutant mapping assay. H and I, deletion of the critical domain allows eIF3k no longer inhibit MyD88-induced NF-kB signaling. EPC cells were transfected with the indicated plasmids. The luciferase activity was measured and normalized to renilla luciferase activity. The data are shown as the mean ± SD of three independent experiments. (∗) p< 0.05, (∗∗) p< 0.01 versus the controls. Co-IP, coimmunoprecipitation; eIF3k, eukaryotic translation initiation factor 3k; EPC, epithelioma papulosum cyprini; HAM, HEAT analog motif; TIR, Toll/interleukin-1 receptor.