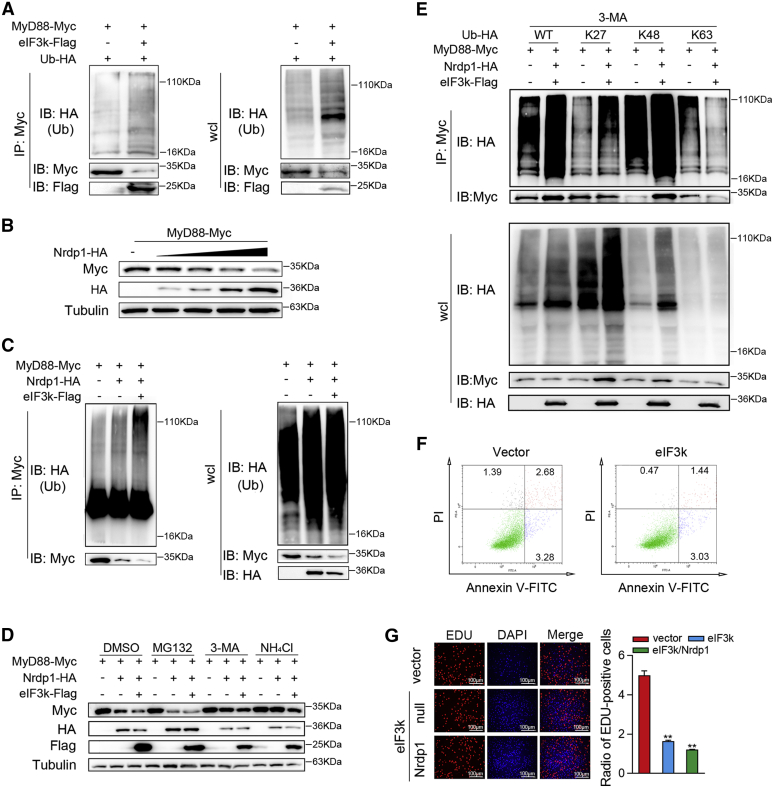
Figure 6.
eIF3k potentiates Nrdp1 ubiquitinate MyD88.A, eIF3k enhances the ubiquitination of MyD88. HEK293 cells were transfected with the indicated plasmids for 32 h, cells were subjected to Co-IP and ubiquitination analysis with the indicated antibodies. B, overexpression of eIF3k decreases the MyD88 protein level. MyD88 were co-transfected with the increased amount of Nrdp1 for 48 h in EPC cells and followed by immunoblot. C, eIF3k potentiates Nrdp1 ubiquitinate MyD88. HEK293 cells were transfected with the indicated plasmids for 32 h; cells were subjected to Co-IP and ubiquitination analysis with the indicated antibodies. D, eIF3k enhances Nrdp1-mediated autophagic degradation of MyD88. EPC cells were transfected with the indicated plasmids for 30 h. Cells were treated with MG132, 3-MA and NH4Cl for an additional 8 h before immunoblot analysis. E, Nrdp1 promote K27-linked ubiquitination of MyD88. HEK293 cells were transfected with the indicated plasmids for 30 h. Cells were treated with 3-MA for 6 h before use in ubiquitination assays. F, apoptosis in eIF3k overexpressing or control MIC cells. Cells were stained with PI and Annexin-V-FITC, and the positive stained cells were counted using FACScan. G, MIC cells were transfected with the indicated plasmids for 36 h before cell proliferation assays. Scale bar, 100 μm. The data are shown as the mean ± SD of three independent experiments. (∗) p< 0.05, (∗∗) p< 0.01 versus the controls. 3-MA, 3-methyladenine; eIF3k, eukaryotic translation initiation factor 3k; EPC, epithelioma papulosum cyprini; MIC, M. miiuy intestine cell.