Abstract
OBJECTIVE
We assessed whether Index60, a composite measure of fasting C-peptide, 60-min C-peptide, and 60-min glucose, could improve the metabolic staging of type 1 diabetes for progression to clinical disease (stage 3) among autoantibody-positive (Ab+) individuals with normal 2-h glucose values (<140 mg/dL).
RESEARCH DESIGN AND METHODS
We analyzed 3,058 Type 1 Diabetes TrialNet Pathway to Prevention participants with 2-h glucose <140 mg/dL and Index60 <1.00 values from baseline oral glucose tolerance tests. Characteristics associated with type 1 diabetes (younger age, greater Ab+, higher HLA DR3-DQ2/DR4-DQ8 prevalence, and lower C-peptide) were compared among four mutually exclusive groups: top 2-h glucose quartile only (HI-2HGLU), top Index60 quartile only (HI-IND60), both top quartiles (HI-BOTH), and neither top quartile (LO-BOTH). Additionally, within the 2-h glucose distribution of <140 mg/dL and separately within the Index60 <1.00 distribution, comparisons were made between those above or below the medians.
RESULTS
HI-IND60 and HI-BOTH were younger, with greater frequency of more than two Ab+, and lower C-peptide levels, than either HI-2HGLU or LO-BOTH (all P < 0.001). The cumulative incidence for stage 3 was greater for HI-IND60 and HI-BOTH than for either HI-2HGLU or LO-BOTH (all P < 0.001). Those with Index60 values above the median were younger and had higher frequency of two or more Ab+ (P < 0.001) and DR3-DQ2/DR4-DQ8 prevalence (P < 0.001) and lower area under the curve (AUC) C-peptide levels (P < 0.001) than those below. Those above the 2-h glucose median had higher AUC C-peptide levels (P < 0.001), but otherwise did not differ from those below.
CONCLUSIONS
Index60 identifies individuals with characteristics of type 1 diabetes at appreciable risk for progression who would otherwise be missed by 2-h glucose staging criteria.
Introduction
Type 1 diabetes has been classified into three distinct stages of disease: stage 1 individuals are presymptomatic, with two or more islet autoantibodies (Ab+) and normal glucose tolerance; stage 2 individuals are presymptomatic, with two or more Ab+ and loss of normal glucose tolerance (i.e., dysglycemia); and stage 3 individuals have clinical diabetes (1). This characterization has established risk profiles for progression to stage 3 among individuals at each stage. Indeed, the risk for progression to stage 3 is increased among individuals in stage 2 (5-year risk of ∼75%) compared with those in stage 1 (5-year risk of ∼44%), highlighting the importance of identifying abnormalities in glucose levels on oral glucose tolerance testing (OGTT) (2,3).
As indicated above, stage 1 is partially defined by the presence of normal glucose levels. However, data suggest that normal glucose levels do not necessarily equate to a normal metabolic state. Glucose values within the normal range have been shown to have a risk gradient for progression to stage 3 in an Ab+ population (4). Also, a Diabetes Prevention Trial Type 1 Risk Score (DPTRS) of ≥7.00 could identify Ab+ individuals within the normal glucose range who were at substantial risk for progression to stage 3 (5). The findings from those studies suggested the possibility that the current metabolic standard for defining stage 1 (i.e., normal glucose levels) could result in misclassification. This has important implications for type 1 diabetes risk assessments in natural history studies and in the selection of participants for prevention trials.
Studies using Index60, a novel composite measure of glucose and C-peptide that has shown promise as an indicator of impending stage 3 among individuals with typical characteristics of type 1 diabetes (6), have raised additional questions about the adequacy of metabolically staging type 1 diabetes according to glucose levels alone in Ab+ individuals. These studies have shown that unless Index60 (or a similar composite glucose and C-peptide measure) is taken into account, individuals at stage 2 or stage 3 can have atypical characteristics for type 1 diabetes (e.g., older, more overweight, lower prevalence of two or more Ab+, and greater C-peptide secretion) (Supplementary Table 1) (7,8).
Building on this work, we hypothesized that combined C-peptide and glucose measures would identify individuals with previously unrecognized metabolic abnormalities and other typical characteristics associated with type 1 diabetes. Thus, we assessed whether Index60 measurements could identify individuals in an Ab+ cohort with normal 2-h glucose values from the Type 1 Diabetes TrialNet Pathway to Prevention study (TNPTP) who had characteristics typically associated with type 1 diabetes (e.g., younger age, increased prevalence of Ab+ and HLA genotypes, and lower measures of C-peptide secretion). We also assessed whether those same individuals would be at appreciable risk for progression to stage 3. Such findings would further support the need to redefine the metabolic staging criteria for type 1 diabetes.
Research Design and Methods
Participants
Data from 3,058 TNPTP participants were analyzed. All had relatives with type 1 diabetes and were between the ages of 1 and 51 years at baseline (for this analysis, baseline was defined as the first OGTT following validation of Ab+). Participants or parents provided written informed consent or assent prior to enrollment, and the study was approved by the respective institutional review board at each site. Participants included in this analysis were found to have either a confirmed single Ab+ (a potential precursor to stage 1) or the presence of two or more diabetes Ab+ (stage 1). A vast majority were found to be Ab+ on initial screening. In the ongoing TNPTP study, Ab+ participants are followed with 2-h OGTT during which glucose and C-peptide measurements are obtained. Individuals with 2-h glucose values <140 mg/dL and Index60 values <1.00 from baseline OGTT were selected for the analyses based on the following reasons. A 2-h glucose threshold of <140 mg/dL has been the standard for a normal 2-h response during OGTT, and it is used for the staging of type 1 diabetes. Also, we found that among TNPTP participants with glucose and Index60 values below the diabetes range (<200 mg/dL and <2.00, respectively), a value of 1.00 had a similar percentile ranking (81.3 percentile) within the Index60 distribution to the ranking of a value of 140 mg/dL within the 2-h glucose distribution (82.7 percentile).
Procedure
The TNPTP has been previously described (9). During the initial screening visit, GAD antibody (GADA), islet antigen 2 antibody (IA-2A), and micro insulin Ab (mIAA) are measured. Individuals determined to have one or more Ab+ are then tested for islet cell cytoplasmic (ICA) and zinc transporter 8 (ZnT8) Ab. The assay for ZnT8 was developed after the inception of the TNPTP; therefore, a sizable proportion of participants did not have ZnT8 measurements at baseline. Ab+ participants are followed with 2-h OGTT for the diagnostic surveillance of stage 3. The 2-h OGTT are performed at 6-month or yearly intervals depending on protocol and risk determination for type 1 diabetes. The 2-h OGTT involve measurements of glucose and C-peptide at 0, 30, 60, 90, and 120 min after the ingestion of 1.75 g/kg carbohydrate (maximum 75 g). Among those who are followed, if a 2-h OGTT is in the diabetes range, a second confirmatory 2-h OGTT is performed. If the confirmatory 2-h OGTT is also in the diabetes range, a diagnosis of type 1 diabetes is made. If the confirmatory 2-h OGTT is not in the diabetes range, 2-h OGTT surveillance continues at 6-month intervals. A diagnosis could also be made clinically without the performance of 2-h OGTT (e.g., symptomatic with marked hyperglycemia). The glucose oxidase method was used to measure plasma glucose. C-peptide was measured by the Tosoh assay. Methods for Ab measurement (10) and HLA genotyping (11) have been described previously. BMI data were calculated and assessed for all participants per Centers for Disease Control and Prevention guidelines and thresholds; BMI percentiles were calculated for all participants age <20 years using Centers for Disease Control and Prevention–based percentiles, whereas BMI percentiles for those age >20 years were imputed using age 20 years and their sex instead of their actual age for the purposes of BMI percentile calculation and corresponding classification (12). Index60 was calculated as previously described based on the following formula: 0.36953 (log fasting C-peptide [ng/mL]) + 0.0165 ∗ glucose60 (mg/dL) − 0.3644 ∗ C-peptide60 (ng/mL), where glucose60 and C-peptide60 are the blood glucose and C-peptide values at 60 min during OGTT, respectively.
Data Analysis
For this analysis, characteristics typically associated with type 1 diabetes and its risk were assessed only in those individuals with normal 2-h glucose values (<140 mg/dL) and Index60 values <1.00. The characteristics associated with type 1 diabetes used in the analysis were age; HLA genotype of DR3-DQ2 and DR4-DQ8; presence of each diabetes autoantibody individually or two or more Ab+(mIAA, IA-2A, or GADA); 30–0 min C-peptide difference (a measure that correlates with first-phase insulin response); and area under the curve (AUC) for C-peptide. Each of these characteristics was measured and/or recorded at either the screening visit or the baseline OGTT. All fasting values were based at time 0 of the OGTT.
For this exploratory study, two primary analyses were performed. In the first, quartiles within normal ranges were established for both 2-h glucose and Index60. Values of 118 mg/dL and 0.32 defined the lower boundaries for the top quartiles of 2-h glucose <140 mg/dL and Index60 <1.00, respectively (Supplementary Tables 2 and 3). Individuals were then divided into four groups based on their 2-h glucose and Index60 values for subsequent analyses (Supplementary Fig. 1): 1) top quartile for 2-h glucose and Index60 below top quartile (HI-2HGLU), 2) top quartile for Index60 and 2-h glucose below top quartile (HI-IND60), 3) top quartiles for both 2-h glucose and Index60 (HI-BOTH), and 4) below top quartiles for both 2-h glucose and Index60 (LO-BOTH).
Each group was thus mutually exclusive; no participant was included in more than one group. The four groups were then compared for characteristics typically associated with type 1 diabetes. Combined glucose and C-peptide response curves from mean OGTT values at 30, 60, 90, and 120 min were plotted on two-dimensional grids to highlight metabolic differences between groups. Mean values for glucose and C-peptide at each time point for all four groups are depicted in Supplementary Table 4.
The other primary analysis compared type 1 diabetes characteristics between those above or below the median within the 2-h glucose distribution of <140 mg/dL and between those above or below the median for the Index60 distribution of <1.00. For comparisons within the distributions, groups were assigned as those less than or equal to the median and those above the median. χ2 tests and t tests were used for comparisons. Assessments of association used linear regression for continuous variables and logistic regression for categorical variables. Proportional hazards regression and log-rank tests were used for stage 3 cumulative incidence analyses.
Results
Among the 3,058 TrialNet participants included in the full study cohort (2-h glucose <140 mg/dL and Index60 <1.00), the baseline mean ± SD age and BMI percentile were 17.9 ± 12.8 years and 63.7 ± 28.5, respectively (Supplementary Table 5). Forty-eight percent were male. There were 66% with a single Ab+ and 34% with two or more Ab+ for GADA, IA-2A, and mIAA.
Comparisons Between Top Quartiles for Characteristics Associated With Type 1 Diabetes
Demographics
Comparisons were made between the four mutually exclusive groups (based on the presence or absence in the top quartiles of 2-h glucose <140 mg/dL or Index60 <1.00) for demographic characteristics (Table 1). HI-IND60 was younger, had lower BMI percentiles, and included a greater percentage of males (P < 0.001 for all) than HI-2HGLU. HI-2HGLU did not significantly differ from LO-BOTH, except for having greater BMI percentiles (P < 0.001). HI-IND60 was somewhat younger than HI-BOTH (P = 0.024), with a tendency toward lower BMI percentiles (P = 0.059), but was otherwise similar.
Table 1.
Demographics for 2-h glucose and Index60 groups
| Feature | HI-2HGLU (A) (n = 533) | HI-IND60 (B) (n = 539) | HI-BOTH (C) (n = 225) | LO-BOTH (D) (n = 1,761) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A vs. D | B vs. D | C vs. D | A vs. B | A vs. C | B vs. C | |||||
| Age at first OGTT | NS | <0.001 | <0.001 | <0.001 | <0.001 | 0.024 | ||||
| Mean ± SD, years | 20.0 ± 13.1 | 10.8 ± 9.9 | 12.6 ± 10.4 | 20.1 ± 12.8 | ||||||
| NR | 2 | 2 | 0 | 2 | ||||||
| Sex | NS | <0.001 | 0.022 | <0.001 | 0.013 | NS | ||||
| Female, n | 302 | 213 | 102 | 967 | ||||||
| Male, n (%) | 228 (43) | 323 (60) | 122 (54) | 788 (45) | ||||||
| NR | 3 | 3 | 1 | 6 | ||||||
| BMI percentile | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | NS | ||||
| Mean ± SD | 71.5 ± 26.8 | 53.8 ± 28.4 | 58.1 ± 29.1 | 65.1 ± 27.9 | ||||||
| NR | 1 | 8 | 1 | 5 | ||||||
NR, not reported; NS, not significant.
Islet Ab
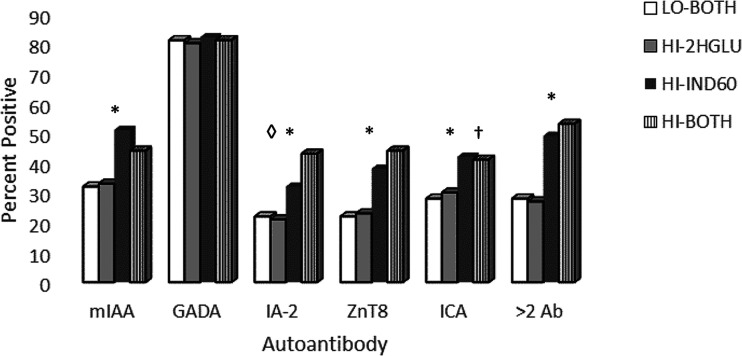
Figure 1 and Supplementary Table 6 show comparisons of Ab+ distribution (mIAA, GADA, IA-2A, ZnT8, ICA, and two or more Ab+) between the four groups. HI-IND60 collectively had substantially greater percentages of positivity than HI-2HGLU for all Ab (P < 0.001), except for GADA, which did not differ significantly between any of the groups. There were no significant differences in the distribution of Ab+ between HI-2HGLU and LO-BOTH. The percentages differed significantly between HI-IND60 and HI-BOTH for ICA, which was higher in the former (P = 0.003), and for IA-2A, which was higher in the latter (P < 0.003). As demonstrated in Fig. 1, HI-2HGLU and LO-BOTH aggregated together with a lower prevalence of two or more Ab+, whereas HI-IND60 and HI-BOTH aggregated together with a higher prevalence.
Figure 1.
Distribution of Ab and measures of C-peptide among groups. Note the consistent aggregation of HI-IND60 with HI-BOTH and of HI-2HGLU with LO-BOTH. *P < 0.01 for greater percentages of all Ab measures in HI-IND60 and HI-BOTH than in either HI-2HGLU or LO-BOTH. †P = 0.003 for greater percentage of ICA in HI-2HGLU than in HI-BOTH. ◊P < 0.003 for greater percentage of IA-2A in HI-BOTH than in HI-IND-60.
Presence of Both DR3-DQ2 and DR4-DQ8
The prevalence of both DR3-DQ2 and DR4-DQ8 was compared among the four groups (Supplementary Table 7). Percentages of positivity for the DR3-DQ2 and DR4-DQ8 combination were higher for HI-IND60 and HI-BOTH (P = 0.002 and P = 0.015, respectively) than for LO-BOTH. Although HI-IND60 tended to have a greater percentage of positivity (20.3%) compared with HI-2HGLU (16.9%), the difference was not statistically significant (P = 0.15).
C-Peptide
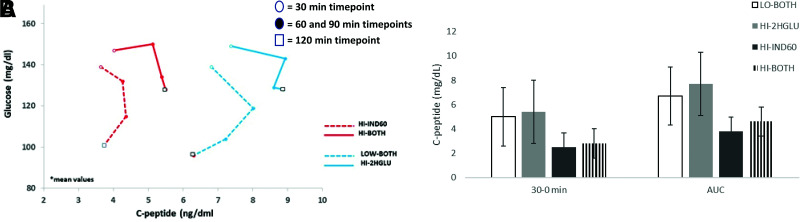
Mean glucose and C-peptide values at each OGTT time point are displayed in Supplementary Table 4. Combined glucose and C-peptide response curves for each of the four groups are shown in Fig. 2A. Evident are the markedly lower C-peptide values in the groups with Index60 in the highest quartile (HI-IND60 and HI-BOTH) compared with the other groups. The difference in C-peptide was significant (P < 0.001) at all OGTT time points for each comparison of HI-IND60 and HI-BOTH with HI-2HGLU or LO-BOTH. Supplementary Table 8 and Fig. 2B show AUC C-peptide and 30–0-min C-peptide values for the groups. Both C-peptide measures were appreciably lower in HI-IND60 than in HI-2HGLU (P < 0.001 for both). LO-BOTH and HI-2HGLU aggregated together with higher C-peptide levels, whereas HI-BOTH and HI-IND60 aggregated together with lower levels. These differences held, even when adjusting for age at the time of the OGTT in the regression models.
Figure 2.
A: Paired C-peptide and glucose values during OGTT among groups. Mean values for glucose and C-peptide are plotted for each of the four groups (HI-IND60, HI-BOTH, LO-BOTH, and HI2HGLU) from the 30- through the 120-min OGTT time points. Open circles represent the plotted intersection of the glucose and C-peptide values at the 30-min time point for each group. Solid circles represent intersections of both values at the successive 60- and then 90-min time points within each group. Lastly, the plotted intersection for glucose and C-peptide at 120 min is represented by the corresponding open square. Each time point within a given group is connected (30 → 60 → 90 → 120 min) to create unique shapes. Note the considerable differences in the shapes. B: C-peptide measures among four top quartile groups. Values were significantly lower for HI-IND60 and HI-BOTH. P < 0.001 for comparisons between each group for both 30–0 min and AUC, except P = 0.022 between LO-BOTH and HI-BOTH for 30–0 min and P = 0.002 between HI-IND60 and HI-BOTH for 30–0 min.
Associations Within the 2-Hour Glucose <140 mg/dL Distribution and Within the Index60 <1.00 Distribution
Assessments of associations within distributions (Supplementary Fig. 2) were consistent with the comparisons between the top quartiles.
Ab Profiles
No differences were observed in percentages of each Ab type or of two or more Ab+ between those above and below the glucose median of the <140 mg/dL 2-h glucose distribution (Supplementary Table 9). In contrast, participants above the median value of the <1.00 Index60 distribution had higher percentages of all Ab+ (except for GADA) and two or more Ab+ compared with those below the median. The Ab differences persisted (P < 0.001) after an adjustment for 2-h glucose in a logistic regression model, indicating that the differences were independent of that measure.
BMI Percentiles
Within the <140 mg/dL 2-h glucose distribution, those above the median had higher BMI percentiles (P < 0.001). In contrast, within the Index60 <1.00 distribution, those above the median had lower BMI percentiles (P < 0.001).
Age
Within the <140 mg/dL 2-h glucose distribution, those above the median were older (P < 0.001), whereas participants above the median within the Index60 <1.00 distribution were younger (P < 0.001).
HLA Genotypes
There was no difference in the prevalence of the DR3-DQ2 and DR4-DQ8 combination between those above or below the median within the <140 mg/dL 2-h glucose distribution. The prevalence was significantly higher for those above the median of the Index60 <1.00 distribution (P < 0.001). The difference persisted (P < 0.001) with the adjustment for 2-h glucose in a logistic regression model.
C-Peptide Response
Within the <140 mg/dL 2-h glucose distribution, mean values for C-peptide AUC and early (30–0 min) C-peptide responses were both increased in those above the median compared with those below (P < 0.001 and P = 0.02, respectively). Conversely, within the Index60 <1.00 distribution, mean C-peptide AUC and 30–0 C-peptide responses were significantly lower in those above the median compared with those below (both P < 0.001).
Comparisons of Cumulative Incidence for Stage 3
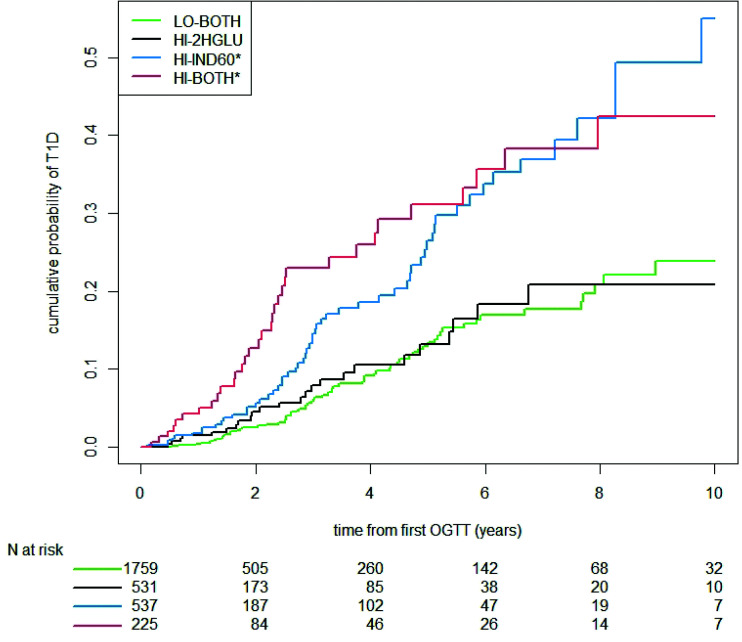
Figure 3 shows cumulative incidence curves of the diagnosis of stage 3 for each of the four mutually exclusive groups. The cumulative incidence curves aggregated together for HI-IND60 and HI-BOTH and were significantly higher (P < 0.001 by log rank) than the curves for HI-2HGLU and LO-BOTH, which also aggregated together. The cumulative incidence of progression to stage 3 was significantly higher for HI-IND60 than for HI-2HGLU, both without (P = 0.001) and with (P = 0.017) adjustments for age and BMI percentile. The cumulative incidence between HI-IND60 and HI-BOTH was not significantly different. In proportional hazards regression models with LO-BOTH as the reference group, the hazard ratios (with 95% CI) were significant for HI-IND60 and HI-BOTH (2.42 [1.70, 3.44] and 2.96 [1.96, 4.47], respectively; P < 0.01 for each after age and BMI percentile adjustments). The hazard ratio was not significant for HI-2HGLU (1.11 [0.69, 1.76]; P = 0.70).
Figure 3.
Cumulative incidence curves for progression to clinical disease within each group. HI-IND60 and HI-BOTH aggregated with higher cumulative incidence than HI-2HGLU or LO-BOTH. *P < 0.001 compared with HI-2HGLU and LO-BOTH. T1D, type 1 diabetes.
The analyses within the Index60 and the 2-h glucose distributions also showed that Index60 was a better predictor of risk than 2-h glucose. The 5-year risk estimates (and corresponding 95% CI) were 0.28 (0.22, 0.33) among those above the 75th percentile (n = 763) of the Index60 distribution and 0.35 (0.24, 0.43) among those above the 90th percentile (n = 303). The 5-year estimate of 0.20 (0.14, 0.25) among those above the 75th percentile (n = 818) of the 2-h glucose distribution decreased to 0.13 (0.07, 0.18) among those above the 90th percentile (n = 320).
Conclusions
In this analysis of Ab+ relatives with 2-h glucose levels <140 mg/dL and Index60 values <1.00, characteristics typical of type 1 diabetes (e.g., younger age, greater prevalence of Ab+ and DR3-DQ2/DR4-DQ8 HLA genotype, and lower C-peptide indices) were more strongly associated with Index60 than with 2-h glucose; there was little if any association of those characteristics with 2-h glucose. In the comparisons between the top quartiles of the distributions, HI-IND60 and HI-BOTH had characteristics more typical of type 1 diabetes than HI-2HGLU. Moreover, the findings were similar between HI-IND60 and HI-BOTH. Also, the cumulative incidence of clinical diabetes (stage 3) was significantly higher for HI-IND60 than for HI-2HGLU and not different between HI-IND60 and HI-BOTH. Individuals in the more typical and higher-risk HI-IND60 and HI-BOTH groups combined represented 25% of individuals (n = 764) in the study cohort of 3,058 with normal glucose levels who otherwise would not have been identified.
In the analysis comparing those above and those below the medians of the <140 mg/dL and Index60 <1.00 distributions, type 1 diabetes characteristics were consistently more associated with Index60 than with 2-h glucose. Within their distributions, Index60 better identified individuals with characteristics of type 1 diabetes than 2-h glucose. Thus, this second analytic approach further demonstrated the potential value of measuring Index60.
The findings for those in HI-2HGLU are analogous to our previous findings for individuals within the dysglycemic range, but with Index60 <1.00 (7). Among them, type 1 diabetes–associated characteristics were less common and the risk for progression to diabetes was lower than those with Index60 values ≥1.00 and normal glucose levels. In a sense, those with dysglycemia were outliers relative to those with higher Index60 values.
The evidence that glucose only groups are outliers was also bolstered in a recent study in which Ab+ individuals in the diabetic range of 2-h glucose at baseline, but with Index60 values <2.00, had fewer characteristic attributes of type 1 diabetes than those with Index60 values ≥2.00 and 2-h glucose levels in the nondiabetic range (8). Thus, in normal, abnormal, and diagnostic ranges of glycemia among Ab+ individuals, Index60 appears to identify individuals with more consistent and similar characteristics. In contrast, higher 2-h glucose levels appear to identify more heterogeneous individuals. Metabolic differences uncovered by indices incorporating measures of endogenous insulin secretion like Index60 could thus help further define diabetes endotypes (13).
It is even possible that Ab+ hyperglycemic individuals with mild C-peptide deficiency do not progress to stage 3 and that they are a separate entity entirely. Some of those individuals might progress to type 2 diabetes or even conceivably remain in a state of dysglycemia. In fact, those disorders would not be unexpected in an Ab+ population, because they are so common in the general population. Indeed, Ab+ adults with diabetes (i.e., adults with latent autoimmune diabetes) are quite atypical with respect to clinical features, severity of insulin deficiency, and progression of disease (14). In children, the terms type 1.5 diabetes, double diabetes, and hybrid diabetes have been coined for those with obesity and a clinical diagnosis of type 2 diabetes who are also positive for islet Ab (15). Given the potential for marked heterogeneity, the inclusion of individuals based upon Ab+ and/or glucose values alone in prevention trials might cloud outcomes.
The findings in this report, together with our prior findings in the dysglycemic and diagnostic ranges, suggest that the current glucose-based staging paradigm for progression to clinical type 1 diabetes (the presence of two or more Ab+, followed by dysglycemia, followed by clinical type 1 diabetes) (1) requires modification. It is apparent that Index60 uncovers metabolic impairment in an appreciable percentage of individuals now being classified as being metabolically normal in stage 1 or even prestage 1 (stage 0; single Ab+). Moreover, individuals classified as being in stage 2 can have atypical characteristics of type 1 diabetes and might even be outside the type 1 diabetes pathogenic pathway. Finally, if a diagnosis is based on glucose thresholds alone, Ab+ individuals could be misclassified at diagnosis as having clinical type 1 diabetes (stage 3). Based on prior findings, designating individuals with Index60 levels ≥2.00 as stage 3 (6,8) seems at least as justified as designating those with 2-h glucose levels ≥200 mg/dL.
These findings raise important questions regarding whether glucose and C-peptide measures from a more abbreviated OGTT (i.e., using measures within or up to 1 h only) would be as or more effective than the 2-h time point in identifying at-risk individuals. Indeed, in an at-risk adult type 2 diabetes population, a 1-h glucose value >155 mg/dL during an OGTT was a better predictor for type 2 diabetes risk progression than the 2-h glucose value or insulin measures at various time points (16). Similarly, within an at-risk Ab+ population, a 1-h glucose value <180 mg/dL had the same predictive capability for progression to disease as a 2-h value <140 mg/dL (17). However, the 1- and 2-h glucose values were both inferior in predicting 3- and 5-year risk compared with measures that incorporated C-peptide (DPTRS and DPTRS60). Similarly, further reducing the needed time points during an OGTT (e.g., using an Index30 measure) to establish this metabolic vulnerability significantly decreases the predictive capability as compared with Index60. Thus, the current use of OGTT-based glucose levels alone, either at the 1- or 2-h time point, without consideration of C-peptide out to 60 min, can be misleading as the sole metabolic indicator for staging the progression to clinical type 1 diabetes.
The 2-h glucose time point was used in these analyses for several reasons. It has long been used as a primary criterion for separating normal from abnormal glucose states, and a majority of Ab+ individuals deemed to have dysglycemia have 2-h glucose levels ≥140 mg/dL. Perhaps most importantly, it is the main diagnostic glucose criterion for type 1 diabetes (18). Among Ab+ individuals, the 2-h glucose diagnostic threshold of ≥200 mg/dL is usually exceeded before the ≥126 mg/dL fasting glucose threshold is reached.
Of interest historically, the cutoff for diagnosing diabetes (2-h glucose >200 mg/dL) was partially based on glucose values from American Indian populations in the U.S. (19). In a 1979 report from the American Diabetes Association, an intermediate-risk group with impaired glucose tolerance, defined as a 2-h glucose value between 140 and 199 mg/dL, represented a stage in the natural history of non–insulin dependent (i.e., type 2) diabetes (20). Abnormal glucose tolerance has been linked to micro- and macrovascular complications (21), highlighting its importance in sequelae of disease within the type 2 diabetes population. What remains in question, however, is the application of these same 2-h glucose thresholds to the diagnosis and assessment of risk in individuals on a path to stage 3 type 1 diabetes.
Each of the characteristics selected for this analysis either has a proposed mechanistic role in the pathogenesis of disease or has been associated with type 1 diabetes. Taken together, they more fully define a typical type 1 diabetes phenotype. Type 1 diabetes is strongly associated with HLA genotypes (22), and products coded by certain HLA alleles appear to be mechanistically involved in the pathologic process (23). Type 1 diabetes and islet Ab are also strongly associated (24–26), although the role of Ab+ in pathogenesis is not clear. The 30–0 min C-peptide difference has been shown to decline during progression to type 1 diabetes in longitudinal analyses (27,28). The inverse association between type 1 diabetes and age is also well documented (18,29), but the pathogenic connection is obscure.
While the association of type 1 diabetes with BMI is not fully clear, obesity is a major predictor of type 2 diabetes. Interestingly, of the four groups we studied, BMI percentile was greatest in HI-2HGLU (72nd percentile), which approaches a common criterion for overweight. We also observed an association of the HI-IND60 group with male sex. Although no sex-specific differences in the prevalence of type 1 diabetes in children are believed to exist (30), adolescents and young adults from European populations with higher background rates for type 1 diabetes tend to be male predominant (31–33). Moreover, a greater increase in the incidence of type 1 diabetes was found among boys compared with girls in the U.S. (34).
This study had some limitations. Type 1 diabetes–associated characteristics were selected for our analysis based on historical, well-established characteristics of the disease, but without a clear understanding of their role in the pathogenesis of type 1 diabetes. However, these features are being used to define type 1 diabetes endotypes (13) and shed light on heterogeneity within and between diabetes types (35). Because baseline OGTT values were obtained only once for each participant, data on the reproducibility of baseline findings were not available. Although the rationales for using quartiles and medians for the analyses were arbitrary, the findings were consistent between the two approaches.
Findings from this study and our prior studies (7,8) should facilitate optimization of type 1 diabetes prevention trial enrollment. Because of the consistency in characteristics between those above prediagnostic Index60 thresholds and those with type 1 diabetes, Index60 could help select populations of normoglycemic and hyperglycemic Ab+ individuals who better align with the mechanistic rationales for preventive treatments. Also, Index60 thresholds could be used as prevention trial end points, both intermediate and perhaps even diagnostic.
In conclusion, two populations of Ab+ individuals have been identified within the standard normal range of 2-h glucose. One population, with relatively high 2-h glucose levels and relatively low Index60 values, has atypical characteristics and a lower risk of progression to stage 3. These individuals will need further study to better understand whether they belong inside or outside the umbrella of type 1 diabetes and its pathogenesis. The other population has relatively high Index60 values within the normal glucose range and has, independent of 2-h glucose levels, characteristics associated with type 1 diabetes and a greater risk of progression to stage 3. These findings indicate that if dysglycemia is the only metabolic criterion for stage 2, a substantial proportion of individuals with normal glucose levels who have deficient β-cell function would not be identified. The inclusion of Index60 as a formal criterion for stage 2 would identify those individuals already at an appreciable level of metabolic risk despite their normal glucose values and increase the number of appropriate candidates for type 1 diabetes prevention trials.
Article Information
Funding. This trial was sponsored by the Type 1 Diabetes TrialNet Study Group, a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085453, U01-DK-0854 61, U01-DK-085466, U01-DK-085499, U01-DK-085504, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-085476, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-106994, U01-DK-107013, U01-DK-107014, and UC4-DK-106993, JDRF, the Victorian State Government Operational Infrastructure Support Program, and the National Health and Medical Research Council Research Institute Infrastructure Support Scheme.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.M.N. conceived the study design, wrote the manuscript, and researched data. M.J.R. contributed to the discussion and reviewed/edited the manuscript. H.I., L.J., E.K.S., J.P., and J.S. reviewed/edited the manuscript. L.B. researched data, performed statistical analysis, and reviewed/edited the manuscript. S.G. researched data, performed statistical analyses, contributed to the discussion, and reviewed/edited the manuscript. J.M.S. conceived the study design, researched data, performed statistical analyses, and wrote the manuscript. All authors reviewed and approved the final submission. B.M.N. and J.M.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16965520.
References
- 1. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Type 1 Diabetes TrialNet Study Group . The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 2013;56:1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sosenko JM, Palmer JP, Greenbaum CJ, et al.; Diabetes Prevention Trial-Type 1 Study Group . Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care 2007;30:38–42 [DOI] [PubMed] [Google Scholar]
- 5. Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Use of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care 2014;37:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan BM, Boulware D, Geyer S, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Dysglycemia and Index60 as prediagnostic end points for type 1 diabetes prevention trials. Diabetes Care 2017;40:1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redondo MJ, Nathan BM, Jacobsen LM, et al.; Type 1 diabetes TrialNet Study Group . Index60 as an additional diagnostic criterion for type 1 diabetes. Diabetologia 2021;64:836–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet natural history study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 10. Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care 2011;34:1785–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pugliese A, Boulware D, Yu L, et al.; Type 1 Diabetes TrialNet Study Group . HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes 2016;65:1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meah FA, DiMeglio LA, Greenbaum CJ, et al.; Type 1 Diabetes TrialNet Study Group . The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 2016;59:1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020;43:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol 2017;13:674–686 [DOI] [PubMed] [Google Scholar]
- 15. Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 16. Peddinti G, Bergman M, Tuomi T, Groop L. 1-hour post-OGTT glucose improves the early prediction of type 2 diabetes by clinical and metabolic markers. J Clin Endocrinol Metab 2019;104:1131–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons KM, Sosenko JM, Warnock M, et al. One-hour oral glucose tolerance tests for the prediction and diagnostic surveillance of type 1 diabetes. J Clin Endocrinol Metab 2020;105:e4094–e4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 19. Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians. Evidence of bimodality in glucose tolerance distributions. Diabetes 1971;20:756–765 [DOI] [PubMed] [Google Scholar]
- 20. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 21. Kahn R. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 22. Noble JA, Valdes AM, Varney MD, et al.; Type 1 Diabetes Genetics Consortium . HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010;59:2972–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 2017;60:1370–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vehik K, Beam CA, Mahon JL, et al.; TrialNet Natural History Study Group . Development of autoantibodies in the TrialNet natural history study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB study. Diabetes 1999;48:460–468 [DOI] [PubMed] [Google Scholar]
- 27. Evans-Molina C, Sims EK, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group . β cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018;3:e120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sosenko JM, Palmer JP, Rafkin LE, et al.; Diabetes Prevention Trial-Type 1 Study Group . Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care 2010;33:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahlquist G; Swedish Childhood Diabetes Study Group . Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 31. Östman J, Lönnberg G, Arnqvist HJ, et al. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med 2008;263:386–394 [DOI] [PubMed] [Google Scholar]
- 32. Wändell PE, Carlsson AC. Time trends and gender differences in incidence and prevalence of type 1 diabetes in Sweden. Curr Diabetes Rev 2013;9:342–349 [DOI] [PubMed] [Google Scholar]
- 33. Blohmé G, Nyström L, Arnqvist HJ, et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia 1992;35:56–62 [DOI] [PubMed] [Google Scholar]
- 34. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redondo MJ, Hagopian WA, Oram R, et al. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia 2020;63:2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]