Optimization of reaction conditions for intramolecular hydroamination of 1ca.
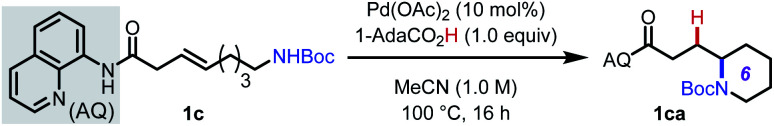
| ||
|---|---|---|
| Entrya | Variation from standard conditions | Yieldb |
| 1 | (None) | 97% (92%) |
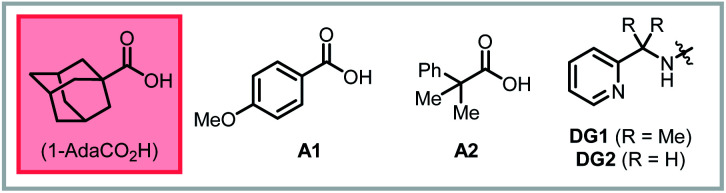
| 2 | AcOH in place of 1-AdaCO2H | 89% |
| 3 | PivOH in place of 1-AdaCO2H | 92% |
| 4 | PhCO2H in place of 1-AdaCO2H | 87% |
| 5 | A1 in place of 1-AdaCO2H | 90% |
| 6 | A2 in place of 1-AdaCO2H | 88% |
| 7 | PhMe as solvent | 82% |
| 8 | PhCN as solvent | 88% |
| 9 | t-AmylOH as solvent | 77% |
| 10 | TFE as solvent | 77% |
| 11 | HFIP as solvent | 65% |
| 12c | 80 °C | 91% |
| 13 | 120 °C | 78% |
| 14 | 0.5 M | 96% |
| 15 | 2.0 M | 90% |
| 16d | DG1 in place of AQ | 44% |
| 17d | DG2 in place of AQ | 16% |
| 18d | OH in place of AQ | n.d. |
| ||
1c (0.1 mmol).
1H NMR yield with CH2Br2 as internal standard; isolated yield given in parentheses.
40 h.
1c (0.05 mmol).
