Gibbs free energies of stationary points in the Pd-catalyzed intramolecular hydroamination of 1a–d. All energy values (in kcal mol−1) are relative to IM1. Bold numbers indicate the most favorable mechanisms in the nucleopalladation step.
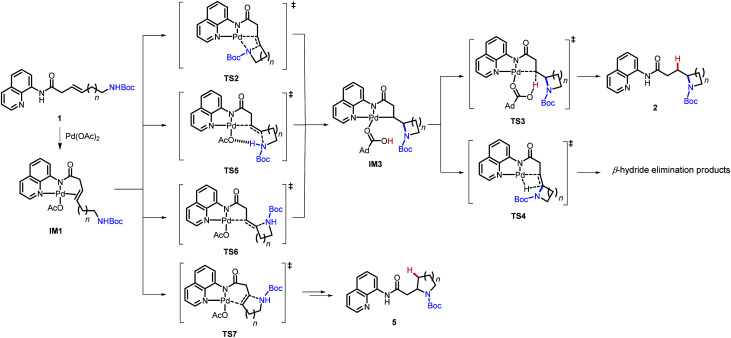
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Alkenyl amine | (n + 3)-Exo-trig | (n + 4)-Endo-trig | (n + 3)-Exo-trig | ||||
| syn-Nucleo-palladation TS | anti-Nucleo-palladation TS | anti-Nucleo-palladation TS | Pallada-cycle | Protode-palladation TS | β-Hydride elimination TS | |||
| TS2 | TS5 | TS6 | TS7 | IM3 | TS3 | TS4 | ||
| 1 | 1a (n = 1) | 31.0 | 29.3 | Cannot locate | 28.0 | 21.8a | 39.6a | 37.6 |
| 2 | 1b (n = 2) | 19.7 | 21.8 | 24.0 | 24.6 | 5.8 | 22.0 | 24.2 |
| 3 | 1c (n = 3) | 23.3 | 21.7 | 24.9 | 32.2 | 5.7 | 22.7 | 23.9 |
| 4 | 1d (n = 4) | 27.1 | 29.1 | 31.3 | Cannot locate | 8.1 | 24.5 | 26.9 |
In the reaction with 1a, the 5-endo-trig regioisomeric pathway is more favorable. The Gibbs free energies of the palladacycle and the protodepalladation TS in the 5-endo-trig pathway with 1a are 9.2 and 29.4 kcal mol−1, respectively.
