FIGURE 2.
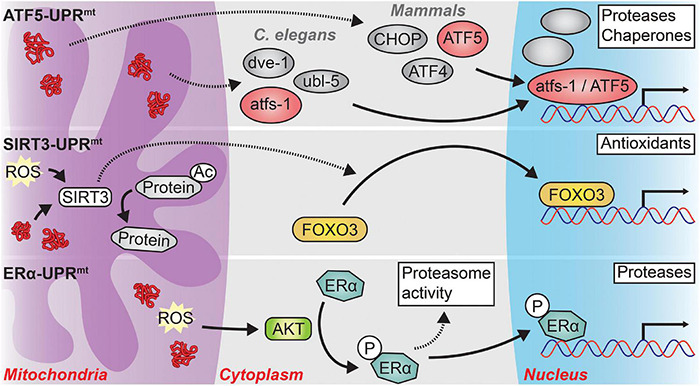
The three major signaling pathways of the UPRmt. In response to mitochondrial stress, three distinct branches of the UPRmt may be activated, depending on the type and location of the mitochondrial stress. ATF5-UPRmt: In C. elegans, protein stress in the mitochondrial matrix causes the cytosolic accumulation of atfs-1, or its mammalian ortholog ATF5. In concert with the transcription factor dve-1 and the ubiquitin-like protein ubl-5, atfs-1 translocates to the nucleus where it induces the transcription of proteases and chaperones to relieve mitochondrial protein stress. A similar process occurs in mammals, albeit with the requirement of two transcription factors, CHOP and ATF4, in addition to ATF5. The precise interactions among atfs-1, dve-1, and ubl-5 as well as among ATF5, CHOP, and ATF4 remain unclear. SIRT3-UPRmt: Mitochondrial matrix reactive oxygen species (ROS) or protein stress activates SIRT3 which then directly deacetylates numerous mitochondrial proteins and indirectly causes the nuclear localization of the transcription factor FOXO3. FOXO3 then induces an antioxidant transcriptional program to combat high levels of oxidative stress in the mitochondria. ERα-UPRmt: Misfolded proteins and ROS located within the mitochondrial intermembrane space (IMS) activate the kinase AKT. AKT phosphorylates Estrogen Receptor alpha (ERα) which then increases the activity of the proteasome and functions as a transcription factor in the nucleus to induce the expression of IMS-specific proteases. Solid arrows represent direct actions while dashed arrows represent indirect actions or actions with unclear mechanisms.
