Abstract
Introduction
Management of traumatic brain injury (TBI) includes invasive monitoring to prevent secondary brain injuries. Intracranial pressure (ICP) monitor is the main measurement used to that intent but cerebral hypoxia can occur despite normal ICP. This study will assess whether the addition of a brain tissue oxygenation (PbtO2) monitor prevents more secondary injuries that will translate into improved functional outcome.
Methods and analysis
Multicentre, randomised, blinded-endpoint comparative effectiveness study enrolling 1094 patients with severe TBI monitored with both ICP and PbtO2. Patients will be randomised to medical management guided by ICP alone (treating team blinded to PbtO2 values) or both ICP and PbtO2. Management is protocolised according to international guidelines in a tiered approach fashion to maintain ICP <22 mm Hg and PbtO2 >20 mm Hg. ICP and PbtO2 will be continuously recorded for a minimum of 5 days. The primary outcome measure is the Glasgow Outcome Scale-Extended performed at 180 (±30) days by a blinded central examiner. Favourable outcome is defined according to a sliding dichotomy where the definition of favourable outcome varies according to baseline severity. Severity will be defined according to the probability of poor outcome predicted by the IMPACT core model. A large battery of secondary outcomes including granular neuropsychological and quality of life measures will be performed.
Ethics and dissemination
This has been approved by Advarra Ethics Committee (Pro00030585). Results will be presented at scientific meetings and published in peer-reviewed publications.
Trial registration number
ClinicalTrials.gov Registry (NCT03754114).
Keywords: protocols & guidelines, adult intensive & critical care, neurological injury, trauma management, neurosurgery, neurophysiology
Strengths and limitations of this study.
BOOST-3, a blinded outcome randomised clinical trial, will determine whether a treatment protocol, informed by brain tissue oxygenation plus intracranial pressure (ICP) monitoring, results in improved neurological outcome measured by the Glasgow Outcome Scale-Extended 6 months after injury compared with treatment guided by ICP monitoring alone.
BOOST-3 is adequately powered to detect a clinically meaningful difference in outcome that remains achievable (10% absolute difference).
The relatively short time window from traumatic brain injury to randomisation (less than 12 hours after injury and 6 hours after presentation at enrolling hospital) will likely reduce generalisability of the findings to underserved communities.
Extensive secondary outcome tests (12 in total) exploring functional and emotional outcome will be performed by blinded centralised examiners.
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in modern industrialised societies.1 The most recent estimates from the Centers for Disease Control and Prevention indicate that in the USA alone, 3.5 million individuals experience a TBI annually, of which 300 000 are hospitalised and discharged alive.2 Among the 300 000 hospitalised survivors, over 40% experience long-term disability.3
Historically, monitoring of patients with severe TBI focused on intracranial pressure (ICP) and cerebral perfusion pressure (CPP) to prevent secondary injury.4–6 Although limiting elevation of ICP is an important part of TBI management, the only randomised controlled trial comparing an ICP-driven management versus clinical management based on imaging and physical examination did not show improvement in outcome with invasive monitoring.7 The management of elevated ICP (eICP) is complex and heterogeneous, this likely reflects the difficulty of applying a one-size-fits-all protocol to a heterogeneous population of patients who require individualised care.8 9
The physiological rationale underlying ICP management is to preserve oxygen delivery to the brain, using CPP as a surrogate for cerebral blood flow (CBF). There are numerous reasons why brain oxygen delivery can be affected despite ICP or CPP being normal.10–12 In fact, oxygen diffusion in the brain parenchyma is the rate limiting step of delivery13 and is affected by the presence of oedema or microcirculatory failure.14 Devices that measure brain tissue oxygen (PbtO2) are now readily available at bedside. Numerous studies have shown that cerebral hypoxia is common, reversible, may be able to measure cerebral ischaemic burden and independently associated with functional outcome.11 15–18 The use of PbtO2 was recently the subject of a consensus statement guideline, highlighting the fact that multimodal monitoring allows for management refinement compared with ICP management alone.19
TBI management heterogeneity requires that any multicentre clinical trial protocol allows various treatment options based on bedside evaluation of cerebral physiology while maintaining the rigour and clinical standardisation necessary to conduct a randomised clinical trial (RCT). BOOST-2, a multicentre RCT, found that treatment of elevated ICP and correction of low PbtO2 decreased the total cumulative ischaemic burden compared with treatment of elevated ICP alone (p=0.0000002).20 Furthermore, a trend in improved functional outcome at 6 months was supportive of the predetermined non-futility hypothesis.
The primary objective of BOOST-3 is to determine whether a treatment protocol, informed by PbtO2 plus ICP monitoring, results in improved neurological outcome measured by the Glasgow Outcome Scale-Extended (GOS-E) 6 months after injury compared with treatment guided by ICP monitoring alone.
Methods
Trial design, study setting and study population
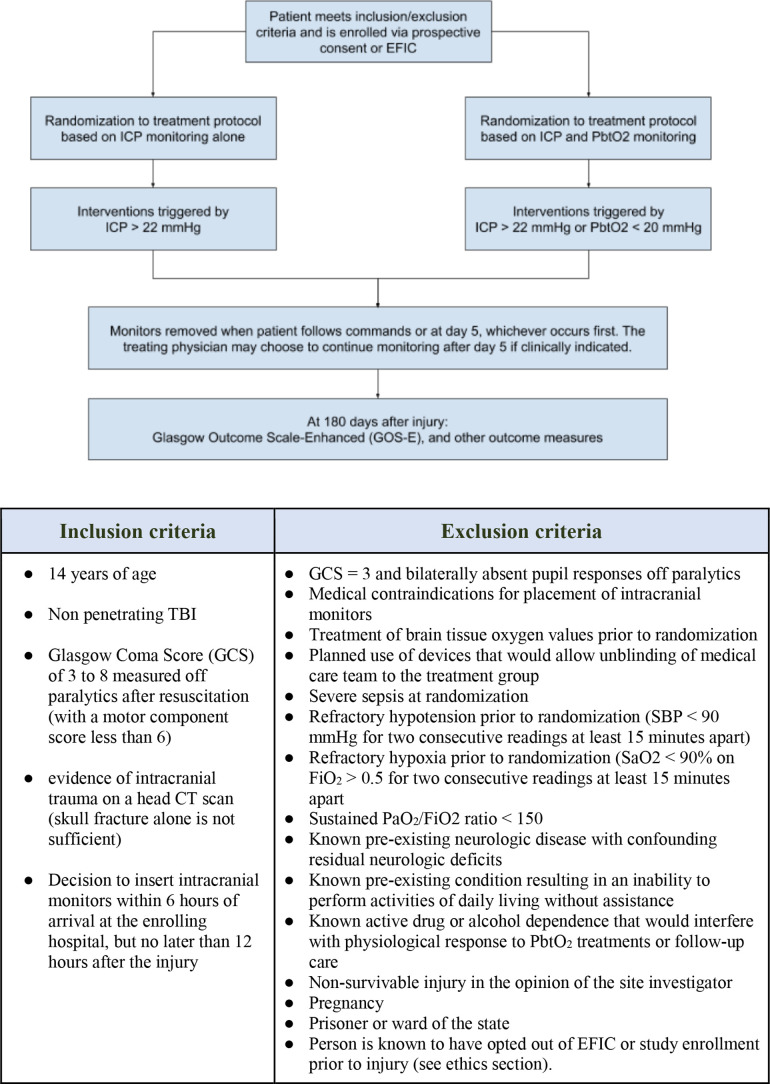
BOOST-3 is a two-arm, single-blind, randomised, controlled, phase III, multicentre trial to determine whether treatment algorithms informed by PbtO2 and ICP monitoring improve subject outcomes more than treatment informed by ICP alone. The complete study protocol, manual of operating procedures (MOP) and other documentation can be found on the study website: siren.network/clinical-trials/boost-3. Inclusion and exclusion criteria are summarised in figure 1.
Figure 1.
Randomisation, inclusion and exclusion criteria. EFIC, exception from informed consent; FiO2, fractional inspired oxygen; ICP, intracranial pressure; PaO2, arterial oxygen pressure; PbtO2, brain tissue oxygenation; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; TBI, traumatic brain injury
BOOST-3 includes 47 level 1 trauma centres that are experienced with active clinical use of PbtO2-guided patient management across the USA and Canada. These sites place PbtO2 and ICP monitors according to Brain Trauma Foundation (BTF) guidelines as part of their standard of care for patients with severe TBI. Monitors will thus be inserted following local standard practice patterns. Of these patients, those who meet eligibility criteria for the study will be randomised. Specifically as per inclusion criteria, randomisation will occur if the decision to palace catheters is made within 6 hours from arrival to the enrolling centre and no later than 12 hours from injury (figure 1).
Both ICP (Codman, Camino or EVD) and PbtO2 monitors (Integra Licox or Raumedic Neurovent) will be used as per local standard practice. Correct catheter placement will be confirmed by a head CT scan within 24 hours of placement. PbtO2 probe reliability will be assessed performing a fractional inspired oxygen (FiO2) challenge (blinded in the ICP-only group) with an appropriate response defined by an increase of at least 5 mm Hg. In the PbtO2+ICP group, non-functioning PbtO2 probes will be replaced.
The trial is being conducted in the SIREN (Strategies to Innovate EmeRgENcy Care Clinical Trials Network) network, which is an emergency care clinical trials network funded by the National Institute for Neurological Disorders and Stroke (NINDS), the National Heart, Lung and Blood Institute and the National Center for Advancing Translational Science to improve outcomes of subjects with acute illness and injury.
Randomisation and blinding
Subjects are randomised in a 1:1 ratio to a treatment protocol informed by both ICP and PbtO2 or by ICP alone, using a covariate-adjusted randomisation scheme (figure 1). The randomisation scheme controls imbalances in the overall treatment distribution, within injury severity category, and within clinical site.
Both arms will have a PbtO2 probe inserted, but the clinical teams will be blinded to PbtO2 values in the ICP-only group. Daily FiO2 challenges will be conducted by unblinded study personnel not involved in patient care to assess probe reliability.
The primary outcome assessment will be centrally performed by trained personnel blinded to group assignment (see the Outcome section).
Intervention
A Clinical Standardization Committee (CST) for the BOOST-3 trial developed general targets for physiological variables for both groups (table 1) and finalised the MOP. Arterial blood pressure monitoring for CPP purposes will be standardised to the level of the heart.
Table 1.
Initial general targets for both groups
| Physiological variable | Desired range |
| Pulse oximetry | >94% |
| PaO2 | >80 mm Hg |
| PaCO2 | 35–45 mm Hg |
| pH | 7.35–7.45 |
| Systolic blood pressure before CPP management | >100 mm Hg if age 50–69 years old >110 mm Hg if age 15–49 or >70 years old |
| Temperature | 36.5°C–37.5°C |
| Maintain normovolaemia | As per local protocol |
| Sodium | 135–145 mmol/L |
| Glucose | 80–180 mg/dL |
| PT and PTT | Normal range as per local hospital guidelines |
| INR | <1.6 |
| Haemoglobin | >70 g/L |
| Platelets for insertion of monitors | >80×109 /L |
CPP, cerebral perfusion pressure; PaCO2, arterial carbon dioxyde pressure; PaO2, arterial oxygen pressure; PT, Prothrombin time; PTT, Pratial Thromboplastine time.
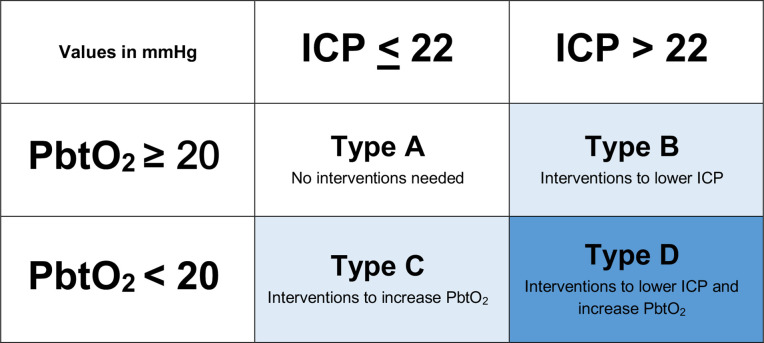
The patient’s clinical course will fall into four different clinical scenarios based on monitoring information, three of which (types B, C and D, defined in figure 2) will require management strategies. Type D combines the treatment options of type B and C scenarios.
Figure 2.
Four possible clinical scenarios based on monitoring information. ICP, intracranial pressure; PbtO2, brain tissue oxygenation.
Scenarios for type B (box 1) and type C (box 2) are addressed with a set of physiologically based interventions to correct ICP and PbtO2. The treatment protocol is tiered in a hierarchical fashion, with less aggressive interventions attempted before more aggressive manoeuvres. Interventions in this protocol were adapted from the BTF 2016 Guidelines for the Management of Severe Traumatic Brain Injury5 and the American College of Surgeons–Trauma Quality Improvement Program 2015 guidelines.6 Some interventions represent expert opinions. Treatment algorithms were developed through discussions between BOOST investigators with expertise in critical care medicine and neurosurgery (CST). The protocol represents an attempt to minimise centre-to-centre variability and to facilitate interpretation of the PbtO2 information using local expertise.
Box 1. Scenario B: treatment options for isolated ICP increase >22 mm Hg.
TIER 1: must begin within 15 min of abnormality. No particular order.
Adjust head of bed to lower ICP.
Ensure temperature <38°C.
Titrate pharmacological analgesia or sedation to effect.
CSF drainage (if EVD available).
Optimise CPP to max 70 mm Hg with fluid boluses or vasopressors as clinically appropriate. May assess cerebral autoregulation as per local protocol to manage CPP targets.
Adjust ventilator for a target PaCO2 of 35–40 mm Hg (target pH of 7.35–7.45).
Low-dose mannitol (0.25–0.5 g/kg).
Low-dose HTS (include 1.5%–3%). This tier does not include higher concentrations of HTS. Titrate to effect (ICP control) and maintain Na <160 mEq/L.
Initiate or titrate anti-epileptic medications.
TIER 2: initiate within 60 min if tier 1 therapies are ineffective. No particular order.
Repeat head CT; treat surgically remediable lesions according to guidelines.
Adjust temperature to 35°C–36°C, using active cooling measures.
NMB, use a bolus dose to determine effect. If effective, perfusion may be used. NMB should be rapidly weaned upon clinical stabilisation.
Optimise CPP: may increase CPP above 70 mm Hg with fluid boluses or vasopressors.*
Adjust ventilatory rate to target PaCO2 of 33–38 mm Hg (target pH of 7.35–7.45).
High-dose mannitol (1–1.5 g/kg) or higher frequency of low-dose mannitol (0.25–0.5 g/kg) if osmolality <320.
High-dose HTS bolus (7.5%, 30 mL of 23.4%). May repeat if Na levels are <160 mEq/L.
TIER 3 (tier 3 therapies are optional). No particular order.
Adjust ventilatory rate for target PaCO2 of 30–35 mm Hg (target pH of less than 7.5).
Pentobarbital coma, according to local protocol. An initial bolus dose of 5 mg/kg should be used to determine effectiveness. If effective, a continuous infusion may be used. Pentobarbital should be rapidly weaned upon clinical stabilisation.
Decompressive craniectomy.
Adjust temperature to 32°C–35°C, using active cooling measures.
Other salvage therapy per local protocol and practice patterns.
*There is a potential for harm related to augmentation of CPP above 70 mm Hg with vasopressors.
CPP, cerebral perfusion pressure; PaCO2, arterial carbon dioxyde pressure; CSF, cerebrospinal fluid; EVD, external ventricular drain; HTS, hypertonic saline; ICP, intracranial pressure; NMB, neuromuscular blockade.
Box 2. Scenario C: treatment options for isolated PbtO2 <20 mm Hg.
TIER 1: must begin within 15 min of abnormality. No particular order.
Adjust head of the bed.
Ensure temperature <38oC.
-
Optimise haemodynamics to ensure adequate CBF and avoid diffusion gradient:
Resuscitation: address hypovolaemia.
Diuresis: avoid hypervolaemia, consider furosemide or other agent for diuresis.
Optimise CPP up to 70 mm Hg maximum with fluid boluses or vasopressors as clinically appropriate. May assess cerebral autoregulation as per local protocol to manage CPP targets.
-
PaO2 adjustment (obtain ABG first*):
Pulmonary toilet with suctioning of secretions (bronchoscopy is not included in this tier as an option).
Increase FiO2 to a maximum of 60%.
Adjust PEEP by a maximum of 5 cm H2O over baseline.
Adjust minute ventilation to achieve a PaCO2 of 38–42 mm Hg (target pH of 7.35–7.45). Further lowering of PaCO2 should not be done if pH >7.45. PaCO2 should not be increased if pH is <7.35.
Initiate or titrate anti-epileptic medications.
TIER 2: initiate within 60 min if tier 1 therapies are ineffective. No particular order.
Increased sedation.
Decrease ICP to <15 mm Hg.
CSF drainage.
NMB, use a bolus dose to determine effect. If effective, perfusion may be used. NMB should be rapidly weaned upon clinical stabilisation.
Optimise CPP: may increase CPP above 70 mm Hg with fluid boluses or vasopressors.
-
PaO2 adjustment (obtain ABG first*):
Perform bronchoscopy.
Increase FiO2 a maximum of 100%†. Wean rapidly when clinically stable (decrease FiO2 by 5% every 30 min).
Adjust PEEP in increments of 3–5 cm H2O.
Adjust minute ventilation to increase PaCO2 to 40–45 mm Hg (target pH of 7.35–7.45).
Transfusion of red blood cells.
TIER 3 (tier 3 therapies are optional). No particular order.
Adjust minute ventilation to increase PaCO2 >45 mm Hg (target pH of 7.30–7.45).
Increase cardiac output with inotropes (milrinone, dobutamine).
Assess for vasospasm with transcranial dopplers, CT angiogram or cerebral angiogram.
Hyperventilation to address possible reverse Robin Hood syndrome.
-
Other potential causes/interventions for low PbtO2 should be considered:
Consider cortical spreading depolarisation via ECog.
Assess for pulmonary embolism.
Assess for cerebral venous thrombosis.
Other salvage therapy based on local protocol and practice patterns.
*Obtain ABG to confirm that oxygenation is in desired range before treating with PaO2 adjustments. Note that increasing PaO2 above 150 mm Hg might imply overtreatment by PaO2 and prevents detection of another potential cause of low PbtO2.
†This option should only be used when PbtO2 is persistently less than 20 mm Hg and other variables contributing to low PbtO2 have been addressed and controlled. There is a potential for harm related to augmentation of CPP above 70 mm Hg with vasopressors.
ABG, arterial blood gas; CBF, cerebral blood flow; CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; FiO2, fractional inspired oxygen; ECog, Electrocorticography; ICP, intracranial pressure; NMB, neuromuscular blockade; PaCO2, arterial carbon dioxyde pressure; PaO2, arterial oxygen pressure; PbtO2, brain tissue oxygenation; PEEP, positive end-expiratory pressure.
An episode that requires intervention is triggered by abnormalities in ICP or PbtO2 lasting more than 5 min. Treatments must be initiated within 15 min of the start of an episode. Patients may start in one type of scenario and then move to another scenario while they are receiving treatments. The initial choice of a treatment option from any tier for any particular scenario should be determined based on what is felt to be the most effective intervention for the current clinical situation, participant characteristics and local protocols. Any intervention chosen should be aimed at addressing the underlying pathophysiology that is contributing to the episode. At least one treatment in tier 1 must be tried before moving on to tier 2. Tier 3 treatments are optional. While there is no maximum number of treatment options that can be attempted from any one tier, no more than 60 min should be spent trying tier 1 interventions prior to moving on to tier 2. The bedside treatment team has the option to progress to higher tiers as rapidly as they feel is clinically indicated.
Some interventions in boxes 1 and 2 are noteworthy.
Optimising CPP
Target range for CPP is unknown and may depend on the patient’s autoregulatory status.4 As such, optimisation of CPP might be informed by cerebral autoregulation testing.21 We advise there is a potential for harm related to augmentation of CPP above 70 mm Hg,22 but some patients may require it. We also recognised that lowering CPP below 60 mm Hg might be an option to treat eICP when cerebral autoregulation is absent (Lund therapy).23 Finally, CPP optimisation also includes improvement in CBF though improvement in cardiac output (inotropy).
Increasing arterial oxygen pressure
Obtaining an arterial blood gas before treating with arterial oxygen pressure (PaO2) adjustments is mandatory. Increasing PaO2 above 150 mm Hg might imply overtreatment by PaO2 and prevents detection of another potential cause of low PbtO2. Calculating the brain oxygen ratio (BOx ratio = PbtO2/PaO2) might help recognise this situation.10 Increasing PaO2 above 150 mm Hg should only be used if PbtO2 is persistently less than 20 mm Hg and other variables contributing to low PbtO2 have been addressed and controlled first.
Reverse Robin Hood syndrome
PbtO2 probe located in an area already maximally vasodilated might measure a drop of flow (low PbtO2) if other areas of the brain vasodilate (potentially because of hypoventilation), creating a ‘steal’ by diverting flow from the area measured. Treatment requires vasoconstricting the normal brain to redirect the flow towards the area measured using hyperventilation.24–26
Withdrawal of life-sustaining treatments (WLST) during the first 5 days will only be considered in dire circumstances or if requested by the patient’s family. If the study subject undergoes WLST during the first 5 days of treatment, the site principal investigator (PI) will be required to notify the study leadership team. Reasons for WLST will be carefully documented.
Outcomes
The primary outcome measure is the GOS-E performed at 180 (±30) days by a blinded central examiner. All injury-related disabilities are assessed for the primary measure.
A complete battery of secondary measures will be assessed, including: survival at hospital discharge, total brain hypoxia burden, Functional Status Examination, Rey Auditory Verbal Learning Test, Trail Making Test Part A and B, Wechsler Adult Intelligence Scale (WAIS-IV), Processing Speed Index, Rivermead Post-Concussion Symptoms Questionnaire, Brief Symptom Inventory 18 and Satisfaction with Life Scale.
Data collection, data monitoring and adverse events
The study data will be managed using the WebDCU system. This web-based clinical trial management system will be used for regulatory document management, subject randomisation, data entry, data validation, project progress monitoring, subject tracking, user customisable report generation and secure data transfer. Reports will be generated to monitor study progress and patient recruitment at each site. These reports will provide centre-specific information on the number of subjects with missing or incomplete data and number of data queries.
Information specific to PbtO2, ICP and CPP monitoring will be collected for up to 5 days. Continuous digital recordings of these values will be captured on a bedside dedicated integrated platform (CNS Monitor, Moberg ICU Solutions, Amber, Pennsylvania, USA). This will allow precise calculation of ischaemic burden (time spent with PbtO2 below 20 mm Hg) and eICP burden (time spent above 22 mm Hg). A custom built-in clinical decision algorithm based on the tier treatments (CNS Carepath, Moberg ICU Solutions, Amber, Pennsylvania, USA) can be used to help guide bedside clinicians to select the appropriate intervention for a given type of scenario. Local study personnel can review Carepath and the medical record to identify alarms and actions taken to correct them on the electronic case report form for the first 5 days.
The clinical site PI, independent medical safety monitor (IMSM), and data and safety monitoring board (DSMB) appointed by the NINDS are responsible for the timely review of the safety data. The DSMB will operate in accordance with NINDS guidelines. The DSMB will evaluate open and closed reports prepared by the Data Coordinating Center on a semiannual basis.
General data quality will be monitored by the Clinical Coordinating Center and will include a combination of on-site monitoring, remote monitoring and central monitoring (using web-based data validation rules, data manager review of entered data, statistical analysis and ongoing review of site metrics).
Adverse events (AEs) are defined as any untoward event or complication not previously identified, or that occurs with greater frequency or severity than previously reported, whether or not considered related to the protocol intervention. The AEs listed in table 2 are anticipated based on the known complications of severe TBI, intracranial monitoring devices and prolonged use of supraphysiological levels of oxygen. In addition, new abnormal laboratory findings that are considered by the treating physician to be clinically significant may be included as AEs.
Table 2.
Adverse event
| Adverse event | Expected incidence |
| Acute respiratory distress syndrome (ARDS) | 5% |
| Pneumonia | 25% |
| Sepsis | 5% |
| Septic shock | 3% |
| Haematoma requiring craniotomy for evacuation | 0.5% |
| Central nervous system infection | <0.5% |
Serious AEs are any AE that results in any of the following outcomes or actions: (1) death due to any cause; (2) a life-threatening adverse experience; (3) inpatient prolongation of existing hospitalisation; (4) a persistent or significant disability/incapacity; (5) an important medical event that may require medical or surgical intervention to prevent one of the outcomes listed above. These must be reported within 24 hours of discovery.
All AEs are collected through day 6 or discharge, whichever comes first; serious AEs will be reported through subject end of study. The IMSM will adjudicate serious AEs for seriousness, relationship to the study intervention and expectedness.
Statistical considerations
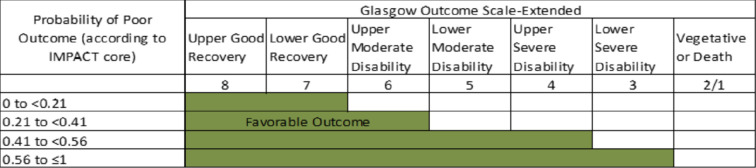
Favourable outcome is defined according to a sliding dichotomy (figure 3),27 where the definition of favourable outcome varies according to baseline severity. Severity will be defined according to the probability of poor outcome predicted by the IMPACT core model.28 The favourable outcome definition is more stringent for subjects with a low probability of poor outcome.
Figure 3.
Outcome defined according to sliding dichotomy.
A clinically relevant effect size of 10% absolute difference in favourable outcome proportions is prespecified. In order to achieve 85% power with a two-sided type I error probability of 0.05, 880 subjects are required. This calculation assumes a 50% favourable outcome proportion in the control arm. Inflation to account for interim analysis and 7% non-adherence results in a maximum sample size of 1094 subjects.
All subjects enrolled in the study are to be followed until the end of study or until consent is withdrawn or declined and will be included in the primary intention-to-treat analysis.
Study timescale
Recruitment began Summer of 2019. The COVID-19 pandemic significantly affected early recruitment. The trial is currently recruiting patients at the rate of 15–16 patients per month. Once all sites are fully operational and recruiting, we expect recruitment to end by 2026. Allowing for the 6-month follow-up assessment, data cleaning and closure of the database, data analyses, manuscript writing and publication should take place in 2026.
Patient and public involvement
Community consultation and public disclosure are completed regionally for all enrolling sites in the USA, prior to the initiation of the clinical trial under CFR 50.24.
No patient or public representative was involved in the written design of the trial.
Ethics and dissemination
Because all patients meeting eligibility criteria for this trial will be unresponsive and unable to provide informed consent, participants will be enrolled either with the informed consent of a legally authorised representative (LAR–see online supplemental material) or with exception from informed consent (EFIC) for emergency research (no EFIC in Canada). If no LAR is available before placement of the ICP and PbtO2 monitors, the patient may be enrolled under EFIC. If LAR is available prior to ICP and PbtO2 monitors being placed, consent will be sought from LAR. The complete EFIC process will be the subject of another publication since it refers to a complex ethical process.
bmjopen-2021-060188supp001.pdf (294.8KB, pdf)
Publication of the results of this trial will be governed by the policies and procedures developed by the Executive Committee consistent with the SIREN publication policy.
Discussion
BOOST-3 is a pragmatic, physiology-based study that aims to demonstrate the superiority of combined PbtO2+ICP-guided therapy over ICP-guided therapy alone when comparing subject outcomes at 6 months. Classic TBI management based on ICP and CPP alone has demonstrated its limitations.29 30 This management uses pressure as a surrogate of CBF and oxygen delivery, an approach that was developed when there was no ability to directly or reliably measure PbtO2.
The development of cerebral hypoxia is now understood to be multifactorial, and at times occurs independent of ICP and CPP abnormalities.11 PbtO2 represents a balance between oxygen delivery and consumption measured directly in the brain parenchyma.31 Analysing the physiological parameters that influence PbtO2 values at the bedside10 allows for a more extensive and precise comprehension of brain pathophysiology and may result in more tailored and efficacious care to prevent secondary injuries.19
Two other trials are going to study the added value of PbtO2 monitoring: the ongoing OXY-TC trial in France32 and the BONANZA trial in New Zealand and Australia (not yet registered on ClinicalTrials.gov). As designed, BOOST-3 will be the largest and is adequately powered to detect a clinically meaningful difference in clinical outcome that remains achievable (10% absolute difference). In comparison, the OXY-TC targets a 30% difference in outcome. Both BOOST-3 and BONANZA will be measuring PbtO2 in a blinded fashion in the control arm allowing the evaluation of cumulative hypoxic burden between groups.
Recognising the heterogeneity of TBI characteristics and complexity of its management, BOOST-3 has standardised therapy in both groups while allowing for flexibility in treatment options. These options reflect the various possible physiological manipulations required to correct anomalies identified by the bedside physician (boxes 1 and 2). Of note, BOOST-3 protocol recognises that cerebral autoregulation status plays an important role in managing CPP threshold.33 Optimisation of CPP according to the autoregulation status might improve outcome but its management remains difficult clinically.21 34–36 PbtO2 might facilitate recognition of the autoregulation status.37 38 Analysis of the continuous data capture within the BOOST-3 cohort may inform future study of the relationship between cerebral autoregulation, goal-directed therapy and patient outcome.
The BOOST-3 protocol also clearly emphasises that increasing PaO2 in order to correct a low PbtO2 value should be used very cautiously. Increasing PaO2 above 150 mm Hg might imply overtreatment by PaO2 and prevents detection of another potential cause of low PbtO2.10 It is possible to compensate for a decrease in PbtO2 due to low CBF by increasing PaO2.39 Hyperoxia is known to induce cerebral vasoconstriction,40 potentially increase free radical production41 and has been associated with worse outcome in other brain ischaemic injuries.42–45 If FiO2 is increased as a therapeutic manoeuvre, a specific FiO2 weaning protocol is suggested. That being said, it is expected that patients with TBI managed with a PbtO2 probe will have a higher mean PaO2 since it is the only possible therapeutic option to address the diffusion and microcirculatory failure often seen with severe TBI.13 14 AEs related to pulmonary pathology will be closely tracked in both study groups.
The limitations of standardisation in BOOST-3 are inherent to the nature of TBI. First, there is wide variation in the phenotype of brain injury. For example, patients may have diffuse axonal injury, intraparenchymal contusion, extra-axial haematomas, subarachnoid haemorrhage or any combination of these injuries.1 The fact that multiparametric and PbtO2 monitoring allow for a physiology-driven approach may globally improve the delivery of care despite the heterogeneity of disease phenotype. BOOST-3 is slated to recruit a large number of patients, which will likely help to achieve balance of injury phenotype across study groups. Furthermore, the specificity gained by measuring functional outcome through a sliding dichotomy based on initial injury should also reduce heterogeneity bias.
WLST, although strongly discouraged in the first 5 days after TBI, can still influence outcome measures. No specific protocol for prognostication and decision to withdraw care is suggested in the research protocol; treating physician acumen will determine end-of-life decisions.
An additional limitation is the relatively short time window from TBI to randomisation (less than 12 hours after injury and 6 hours after presentation at enrolling hospital), this will likely reduce generalisability of the findings to underserved communities, or those lacking access to neurosurgical expertise. This time frame was chosen to appropriately test the biological basis of PbtO2 monitoring in the acute phase of brain injury to prevent secondary injuries. A longer interval from injury may allow for significant cerebral hypoxia before randomisation. A challenge that has been identified after start-up relates to the 6-hour time window after arrival at enrolling site, which poses a problem if the patient needs urgent surgical intervention. Allowing some flexibility in the 6-hour window allows urgent clinical needs to be addressed prior to placement of intracranial monitors. A final challenge after study start-up included the COVID-19 pandemic putting a hold on research activities thus lowering expected enrolment.
The annual cost to society resulting from TBI has been estimated to range from $83 billion to $244 billion (in 2014 dollars).46 Improvements in functional outcome will benefit not only affected patients but society globally. Multiple trials targeting a specific medication or pathophysiological mechanism have failed to demonstrate improvement in outcome so far.47 We feel that the early use of a PbtO2-guided bundle of care will yield a different result.
Supplementary Material
Acknowledgments
We want to acknowledge the influence that the BOOST-3 Clinical Standardization Committee had on developing the clinical application of the protocol: Lori Shutter, Lisa Merck, Ramon Diaz-Arrastia, Rocco Armonda, Francis Bernard, Randall Chesnut, Anita Fetzick, Claude Hemphill, Luke James, Ryan Kitagawa, Carol Moore, David Okonkwo, Ava Puccio, Claudia Robertson, Uzma Samadani, Danielle Sandsmark, Robert Silbergleit.
Footnotes
Contributors: RD-A wrote the protocol of this study. LAS, RD-A, SY and WB are the principal investigators of the trial and compose the steering committee. SY is responsible for the statistics and data management of the trial. WB is administering the trial. FB and LAS wrote the first draft of this manuscript. FB, LAS and LHM are part of the clinical standardisation team responsible for protocol implementation and the manual of operating procedure. FB, LAS, LHM, RD-A, SY and WB all revised and approved the final version of this manuscript.
Funding: This trial is funded by the National Institute of Neurological Disorders and Stroke (NINDS) (grant number U01 NS099046).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- 2.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995-2009. J Safety Res 2012;43:299–307. 10.1016/j.jsr.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Selassie AW, Zaloshnja E, Langlois JA, et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008;23:123–31. 10.1097/01.HTR.0000314531.30401.39 [DOI] [PubMed] [Google Scholar]
- 4.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons . Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24:S1–106. 10.1089/neu.2007.9999 [DOI] [PubMed] [Google Scholar]
- 5.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6–15. 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 6.Cryer H, Manley G, Adelson D. Acs TQIP best practices in the management of traumatic brain injury, 2015. [Google Scholar]
- 7.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med Overseas Ed 2012;367:2471–81. 10.1056/NEJMoa1207363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesnut RM, Petroni G, Rondina C. Intracranial-Pressure monitoring in traumatic brain injury. N Engl J Med 2013;368:1751–2. [DOI] [PubMed] [Google Scholar]
- 9.Le Roux P. Intracranial pressure after the best trip trial: a call for more monitoring. Curr Opin Crit Care 2014;20:141–7. 10.1097/MCC.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 10.Dellazizzo L, Demers S-P, Charbonney E, et al. Minimal PaO2 threshold after traumatic brain injury and clinical utility of a novel brain oxygenation ratio. J Neurosurg 2018:1–9. 10.3171/2018.5.JNS18651 [DOI] [PubMed] [Google Scholar]
- 11.Chang JJJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med 2009;37:283–90. 10.1097/CCM.0b013e318192fbd7 [DOI] [PubMed] [Google Scholar]
- 12.Gagnon A, Laroche M, Williamson D, et al. Incidence and characteristics of cerebral hypoxia after craniectomy in brain-injured patients: a cohort study. J Neurosurg 2020;1:1–8. 10.3171/2020.6.JNS20776 [DOI] [PubMed] [Google Scholar]
- 13.Menon DK, Coles JP, Gupta AK, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med 2004;32:1384–90. 10.1097/01.CCM.0000127777.16609.08 [DOI] [PubMed] [Google Scholar]
- 14.Veenith TV, Carter EL, Geeraerts T, et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury. JAMA Neurol 2016;73:542–50. 10.1001/jamaneurol.2016.0091 [DOI] [PubMed] [Google Scholar]
- 15.Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med 2009;37:2057–63. 10.1097/CCM.0b013e3181a009f8 [DOI] [PubMed] [Google Scholar]
- 16.Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg 2010;113:571–80. 10.3171/2010.1.JNS09506 [DOI] [PubMed] [Google Scholar]
- 17.Weiner GM, Lacey MR, Mackenzie L, et al. Decompressive craniectomy for elevated intracranial pressure and its effect on the cumulative ischemic burden and therapeutic intensity levels after severe traumatic brain injury. Neurosurgery 2010;66:1111–9. 10.1227/01.NEU.0000369607.71913.3E [DOI] [PubMed] [Google Scholar]
- 18.Oddo M, Levine JM, Mackenzie L, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery 2011;69:1037–45. 10.1227/NEU.0b013e3182287ca7 [DOI] [PubMed] [Google Scholar]
- 19.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med 2020;46:919–29. 10.1007/s00134-019-05900-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med 2017;45:1907–14. 10.1097/CCM.0000000000002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly J, Czosnyka M, Adams H, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med 2017;45:1464–71. 10.1097/CCM.0000000000002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med 1999;27:2086–95. 10.1097/00003246-199910000-00002 [DOI] [PubMed] [Google Scholar]
- 23.Nordström C-H. Physiological and biochemical principles underlying volume-targeted therapy--the "Lund concept". Neurocrit Care 2005;2:083–96. 10.1385/NCC:2:1:083 [DOI] [PubMed] [Google Scholar]
- 24.Alexandrov AV, Sharma VK, Lao AY, et al. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke 2007;38:3045–8. 10.1161/STROKEAHA.107.482810 [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov AV, Nguyen HT, Rubiera M, et al. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke 2009;40:2738–42. 10.1161/STROKEAHA.109.547950 [DOI] [PubMed] [Google Scholar]
- 26.Sharma VK, Teoh HL, Paliwal PR, et al. Reversed Robin Hood syndrome in a patient with luxury perfusion after acute ischemic stroke. Circulation 2011;123:e243–4. 10.1161/CIRCULATIONAHA.110.972000 [DOI] [PubMed] [Google Scholar]
- 27.Maas AIR, Murray GD, Roozenbeek B, et al. Advancing care for traumatic brain injury: findings from the impact studies and perspectives on future research. Lancet Neurol 2013;12:1200–10. 10.1016/S1474-4422(13)70234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the impact study. J Neurotrauma 2007;24:329–37. 10.1089/neu.2006.0035 [DOI] [PubMed] [Google Scholar]
- 29.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012;367:2471–81. 10.1056/NEJMoa1207363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stocchetti N, Poole D, Okonkwo DO. Intracranial pressure thresholds in severe traumatic brain injury: we are not sure : Prudent clinical practice despite dogma or nihilism. Intensive Care Med 2018;44:1321–3. 10.1007/s00134-018-5251-4 [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal G, Hemphill JC, Sorani M, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med 2008;36:1917–24. 10.1097/CCM.0b013e3181743d77 [DOI] [PubMed] [Google Scholar]
- 32.Payen J-F, Richard M, Francony G, et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: the multicentre randomised controlled OXY-TC trial study protocol. BMJ Open 2020;10:e040550. 10.1136/bmjopen-2020-040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depreitere B, Güiza F, Van den Berghe G, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 2014;120:1451–7. 10.3171/2014.3.JNS131500 [DOI] [PubMed] [Google Scholar]
- 34.Howells T, Elf K, Jones PA, et al. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg 2005;102:311–7. 10.3171/jns.2005.102.2.0311 [DOI] [PubMed] [Google Scholar]
- 35.Preiksaitis A, Krakauskaite S, Petkus V, et al. Association of severe traumatic brain injury patient outcomes with duration of cerebrovascular autoregulation impairment events. Neurosurgery 2016;79:75–82. 10.1227/NEU.0000000000001192 [DOI] [PubMed] [Google Scholar]
- 36.Jaeger M, Dengl M, Meixensberger J, et al. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med 2010;38:1343–7. 10.1097/CCM.0b013e3181d45530 [DOI] [PubMed] [Google Scholar]
- 37.Lang EW, Czosnyka M, Mehdorn HM. Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit Care Med 2003;31:267–71. 10.1097/00003246-200301000-00042 [DOI] [PubMed] [Google Scholar]
- 38.Jaeger M, Schuhmann MU, Soehle M, et al. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med 2006;34:1783–8. 10.1097/01.CCM.0000218413.51546.9E [DOI] [PubMed] [Google Scholar]
- 39.Hlatky R, Valadka AB, Gopinath SP, et al. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J Neurosurg 2008;108:53–8. 10.3171/JNS/2008/108/01/0053 [DOI] [PubMed] [Google Scholar]
- 40.Floyd TF, Clark JM, Gelfand R, et al. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 Ata. J Appl Physiol 2003;95:2453–61. 10.1152/japplphysiol.00303.2003 [DOI] [PubMed] [Google Scholar]
- 41.Reinert M, Schaller B, Widmer HR, et al. Influence of oxygen therapy on glucose-lactate metabolism after diffuse brain injury. J Neurosurg 2004;101:323–9. 10.3171/jns.2004.101.2.0323 [DOI] [PubMed] [Google Scholar]
- 42.Elmer J, Scutella M, Pullalarevu R, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med 2015;41:49–57. 10.1007/s00134-014-3555-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C-H, Chang W-T, Huang C-H, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation 2014;85:1142–8. 10.1016/j.resuscitation.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 44.Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation 2011;123:2717–22. 10.1161/CIRCULATIONAHA.110.001016 [DOI] [PubMed] [Google Scholar]
- 45.Youn CS, Park KN, Kim SH, et al. The cumulative partial pressure of arterial oxygen is associated with neurological outcomes after cardiac arrest treated with targeted temperature management. Crit Care Med 2018;46:e279–85. 10.1097/CCM.0000000000002935 [DOI] [PubMed] [Google Scholar]
- 46.Corso P, Finkelstein E, Miller T, et al. Incidence and lifetime costs of injuries in the United States. Inj Prev 2006;12:212–8. 10.1136/ip.2005.010983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Arrastia R, Kochanek PM, Bergold P, et al. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of defense neurotrauma pharmacology Workgroup. J Neurotrauma 2014;31:135–58. 10.1089/neu.2013.3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060188supp001.pdf (294.8KB, pdf)