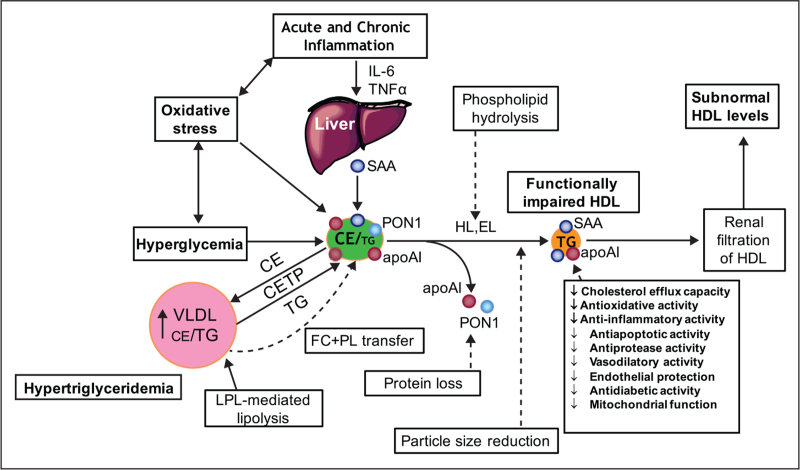
FIGURE 4.
Altered metabolism of HDL in type 2 diabetes with hyperglycaemia and insulin resistance, hypertriglyceridemia and acute and/or chronic systemic inflammation associated with elevated oxidative stress. These factors act in a synergistic manner to modify the lipidome and proteome of HDL particles, resulting in impaired functionality. Chronic inflammation characteristic of type 2 diabetes is associated with elevated plasma levels of IL-6. As a result, the liver and other tissues may produce serum amyloid A, which binds to HDL, displaces apoA-I, paraoxonase-1 and other protein components. Oxidative stress may lead to oxidation of both lipids and proteins. Hyperglycaemia, through the formation of advanced glycosylation end products, results in glycation of HDL proteins, including apoAI, with impaired biological activity. Elevated lipid transfer activity of CETP in hypertriglyceridemia enhances the triglyceride content of HDL with depletion of cholesteryl esters. Attenuated transfer of surface lipolytic fragments of VLDL to the HDL pool occurs in T2DM; these fragments consist mainly of free cholesterol and phospholipids. Lipolysis of HDL lipids by hepatic and endothelial lipases produces small, dense HDL with impaired functionality. Small HDLs are filtered by the kidney with subsequent fall in circulating HDL levels.