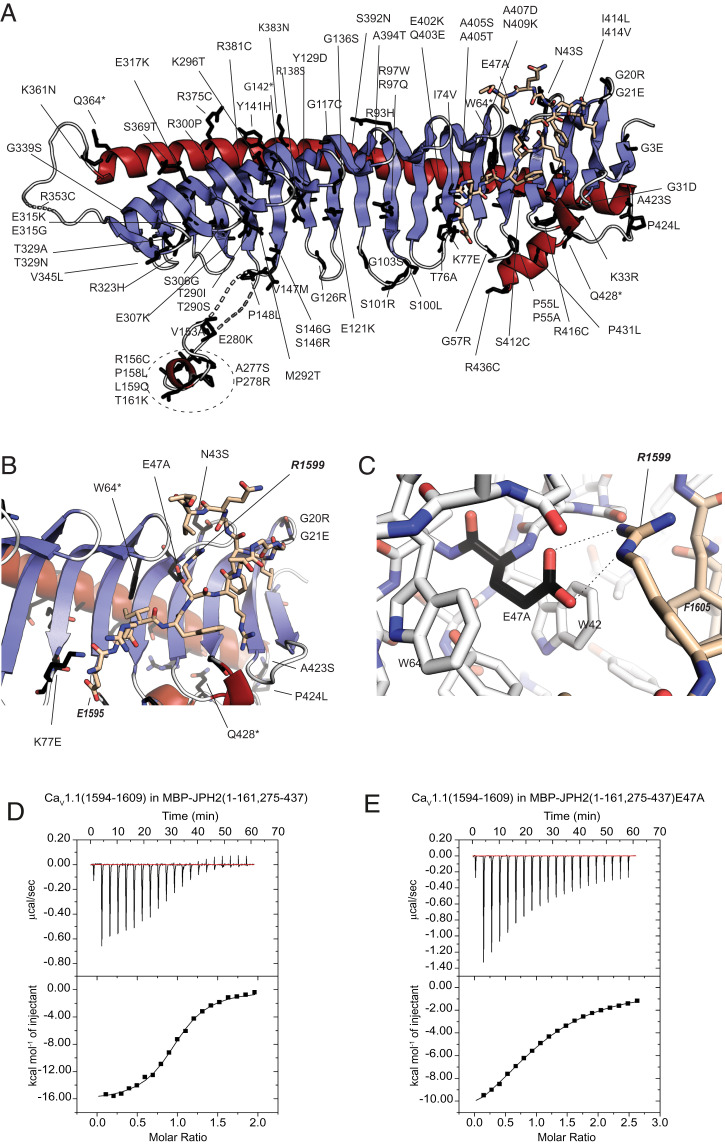
Fig. 4.
Sequence variants in JPH2. (A) JPH2 sequence variants (labeled) are mapped to the crystallized JPH2 MORN-helical domain in complex with the CaV1.1 peptide. Affected residues are shown as black sticks. (B and C) Mutants in proximity to the peptide binding site, with residue E47 from JPH2 forming an ionic interaction with R1599 of the CaV1.1 peptide. (D) A representative ITC of 300 μM CaV1.1 peptide titrated into 35 μM MBP-tagged JPH2 (1 to 161, 275 to 437) (Kd = 1.8 μM). (E) A representative ITC of 750 μM CaV1.1 peptide titrated into 60 μM into the same MBP-tagged JPH2 construct carrying the E47A mutation (Kd = 26 μM).