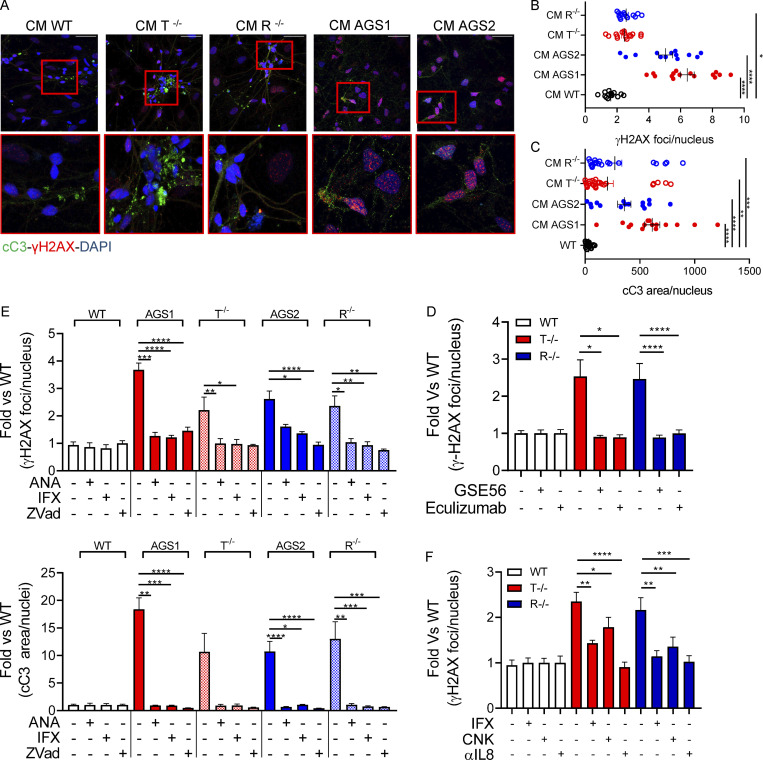
Figure 7.
KO and AGS patient iPSC–derived astrocytes mediate neurotoxicity. (A–C) WT neurons expression of cleaved Caspase-3 (cC3) and phosphorylated γH2AX after 48-h exposure to KO iPSC–derived astrocyte conditioned medium, measured by IF staining. Representative pictures and quantification. Each dot corresponds to one field from three slides of three independent differentiations. Scale bar, 50 µm. (Mean ± SEM; n = 3 independent experiments; Kruskal–Wallis test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.) CM, conditioned medium. (D) Reduced γH2AX upon exposure of WT neurons to conditioned medium collected from WT and TREX1- or RNASEH2B-deficient astrocytes and then supplemented with Complement C5 inhibitor eculizumab or conditioned medium collected from WT and TREX1- or RNASEH2B-deficient astrocytes overexpressing dominant-negative p53-inhibiting peptide GSE56. (Mean ± SEM; n = 3 independent experiments; one-tailed Mann–Whitney U test; *, P < 0.05; ****, P < 0.0001.) (E) Reduced γH2AX and cleaved Caspase-3 immunopositive area upon exposure of WT neurons to conditioned medium collected from astrocytes treated with anakinra (ANA, IL-1β inhibitor), infliximab (IFX, anti-TNFα antibody), or Z-Vad (pan-caspase inhibitor) for 24 h. γH2AX and cleaved Caspase-3 levels expressed as over WT condition. (Mean ± SEM; n = 3 independent experiments; Kruskal–Wallis test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.) (F) Reduced γH2AX immunopositive area upon exposure of WT neurons to conditioned medium collected from astrocytes supplemented with anti-TNFα (infliximab, IFX), anti–IL-1β (canakinumab, CNK), or anti-IL8 for 48 h. γH2AX expressed as fold over WT condition. (Mean ± SEM; n = 3 independent experiments; one-tailed Mann–Whitney U test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.)