Abstract
Introduction:
Several months into the coronavirus disease 2019 (COVID-19) pandemic, there remains a paucity of data on the behavior of the disease in patients with end-stage kidney disease (ESKD) on maintenance hemodialysis (MHD). Here, we describe the clinical presentations, biochemical profile, and outcomes of 183 such patients from a large tertiary-care center in South India.
Materials and Methods:
This prospective, observational study, included all patients with COVID-19 and ESKD who received at least one session of hemodialysis at our center, from the start of the outbreak to July 9, 2020. Clinical features at presentation, laboratory and radiological data, and outcomes were analyzed.
Results:
A total of 183 patients were included in the analysis. Patients who had symptoms at presentation accounted for 49.18% of the cohort, with the most common symptoms being fever (87.1%), cough (67.7%), and breathlessness (63.4%). Factors independently associated with mortality on univariate analysis included age ≥60 years, having symptoms at presentation, neutrophil–lymphocyte ratio >6, C-reactive protein >20 mg/L, serum lactate dehydrogenase >250 IU/L, CT (computed tomography) Grades 3 and 4, and the need for respiratory support. However, on multivariate logistic regression analysis, the only factor that retained significance was an age >60 years.
Conclusions:
This analysis confirms the previous reports of higher COVID-19-related mortality in the dialysis population and identifies older age, higher inflammatory markers, and greater degrees of radiological lung involvement to correlate with increased mortality.
Keywords: COVID-19, ESKD, hemodialysis
Introduction
Coronavirus disease 2019 (COVID-19) was formally declared a public health emergency of international concern on January 30, 2020, by the Director General of the World Health Organization.[1] Since then, the impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has been felt across the globe. Although the disease has been well-characterized in the general population by numerous large-scale studies,[2,3,4] similar data in the dialysis population have been limited to small case series.[5,6,7] The city of Chennai, India, has been particularly hard-hit, accounting for more than 94,000 documented cases as on July, 1, 2020.[8] Because of the limited number of centers with facilities for dialyzing patients with COVID-19 infections, our institution has served as the primary referral center for patients requiring hemodialysis. We now present an analysis of our data, describing the clinical presentations and outcomes of patients with end-stage kidney disease (ESKD) admitted with COVID-19.
Materials and Methods
This is an observational, prospective study conducted at our center, a tertiary-care referral hospital in South India. The study was approved by the Institutional Ethics Committee. All patients with ESKD and COVID-19, who were given at least one session of hemodialysis at our institute, were serially included in the analysis, since the beginning of the outbreak, until the time of writing (July 9, 2020). The patients were required to have a positive nasopharyngeal swab for SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) in order to be included.
In-patient management protocol
As per institutional protocol, all patients with COVID-19 requiring kidney replacement therapy (KRT) were admitted irrespective of symptomatology. Routine laboratory parameters that were measured on admission included a complete blood count and serum biochemistry (including electrolytes, C-reactive protein, ferritin, and lactate dehydrogenase). All patients underwent chest imaging by computed tomography (CT). The findings were graded radiologically based on the percentage of lung involvement (<25%, 25%–50%, 50%–75%, and >75% lung involvement was assigned as Grades 1, 2, 3, and 4, respectively).
Because of logistical limitations, hemodialysis could not be provided by predetermined schedules, and it was performed at the discretion of the treating physician based on clinical and laboratory indications. Patients who were hypoxemic were preferentially given more intensive dialysis with more aggressive ultrafiltration, because of the difficulty in differentiating pulmonary congestion from COVID-19-related pneumonia.
Specific therapy included the use of low-molecular-weight heparin (LMWH; enoxaparin 40 mg subcutaneously daily for 5 days) and steroids for all patients who were hypoxemic despite adequate ultrafiltration, and those with radiological lung involvement of >25% (Grade 2 and above). LMWH was skipped on the morning of dialysis, and standard intradialytic anticoagulation with unfractionated heparin (2500 units [U] bolus, followed by 750 U/hour infusion) was given throughout the session. LMWH was then restarted the next morning. Steroids were initially administered in the form of intravenous (IV) methylprednisolone 1 mg/kg once daily for 5 days in accordance with the local guidelines at the time,[9] but this was later switched to IV dexamethasone 6 mg once daily for 5 days on the basis of newly available trial data.[10] None of the patients in our cohort received antivirals, tocilizumab, or plasma therapy. For all patients with CT Grades 3 and 4, a third general cephalosporin antibiotic was also added to cover bacterial superinfections, especially in the setting of hyperglycemia and steroid use.
Nasopharyngeal swabs for SARS-CoV-2 by RT-PCR were repeated every 72 hours after admission, until a negative result occurred. Criteria for discharge included clinical recovery along with a single negative nasopharyngeal swab for SARS-CoV-2.
Data sources and variables
Information relating to symptoms at presentation, history relating to preexisting conditions and details of dialysis, along with need for respiratory support were all collected by direct patient interviews and clinical assessment at the time of admission. Laboratory and radiology reports were obtained from the daily consolidated data sheets maintained by the Departments of Internal Medicine and Radiology, respectively. Follow-up phone calls were made 2 weeks after discharge, and vital status was ascertained.
Statistical methods
Statistical analysis was performed using IBM® SPSS® Statistics Version 23. Qualitative variables are expressed as number and percentage. Quantitative variables are expressed as mean ± SD or as median (interquartile range [IQR]). Pairwise deletion of missing data was performed during analysis.
Appropriate tests for statistical significance were used for comparisons between symptomatic and asymptomatic individuals – the Chi-squared test or Fisher's exact test for qualitative data, and the independent-samples t test or Mann–Whitney U test for quantitative data. Univariate analysis was performed to identify factors that could predict the risk of death, and all statistically significant predictors were entered into a multivariate logistic regression. A two-sided P value <0.05 was considered to be statistically significant.
Results
Patients and clinical characteristics
A total of 183 consecutive patients with COVID-19 were dialyzed at our center between April 17, 2020, and July 9, 2020. The mean age of the patients was 49.97 years (range: 19–85 years), with 64.5% being male. Detailed descriptions of the patients’ clinical characteristics are presented in Table 1. All patients had chronic kidney disease stage 5D (CKD 5D); the median dialysis vintage was 18 months (IQR: 6–36 months). About 10.4% had coexistent viral infections – hepatitis B (n = 6), hepatitis C (n = 9), hepatitis B and C coinfection (n = 3), and HIV (human immunodeficiency virus; n = 1).
Table 1.
Baseline characteristics, compared with patients who were symptomatic and asymptomatic at presentation
| Variables | All patients (n=183) | Symptomatic (n=93) | Asymptomatic (n=90) | P |
|---|---|---|---|---|
| Age | 49.97±12.99 | 50.14±12.43 | 49.79±13.62 | 0.856 |
| Male | 118 (64.5%) | 57 (61.3%) | 61 (67.8%) | 0.359 |
| Coexisting conditions | ||||
| Hypertension | 140 (76.5%) | 71 (76.3%) | 69 (76.7%) | 0.959 |
| Diabetes mellitus | 62 (33.9%) | 30 (32.3%) | 32 (35.6%) | 0.643 |
| Heart failure | 45 (24.6%) | 20 (21.5%) | 25 (27.8%) | 0.325 |
| COPD/asthma | 11 (6%) | 4 (4.3%) | 7 (7.8%) | 0.323 |
| Laboratory features | ||||
| NLR | 3.2 (2.21-5.9) | 3.2 (2.18-6.54) | 3.2 (2.31-5.66) | 0.880 |
| CRP (mg/L) | 27.6 (7.4-93) | 56.8 (13.4-105) | 13.65 (4.95-43.32) | <0.001 |
| LDH (IU/L) | 288 (209-387) | 321 (219.5-416) | 257.5 (203.5-344.25) | 0.011 |
| Ferritin (ng/mL) | 1,092 (519.5-2,000) | 1,395 (668-2,000) | 808 (449.75-1,700) | 0.003 |
| Imaging findings | ||||
| Not suggestive of COVID-19 | 81 (0.44.5%) | 31 (33.3%) | 50 (56.2%) | <0.001 |
| Grade 1 | 49 (26.9%) | 20 (21.5%) | 29 (32.6%) | |
| Grade 2 | 28 (15.4%) | 22 (23.7%) | 6 (6.7%) | |
| Grade 3 | 15 (8.2%) | 14 (15.1%) | 1 (1.1%) | |
| Grade 4 | 9 (4.9%) | 6 (6.5%) | 3 (3.4%) | |
| Dialysis details | ||||
| Dialysis access | ||||
| Permanent access | 139 (76%) | 66 (71%) | 73 (81.1%) | 0.108 |
| Temporary HD catheter | 43 (23.5%) | 26 (28%) | 17 (18.9%) | 0.148 |
| Dialysis vintage (months) | 18 (6-36) | 16 (6-36) | 24 (6.75-39) | 0.152 |
| Mean dialysis sessions | 2 (1-3) | 2 (2-3) | 2 (1-3) | 0.136 |
| Outcomes | ||||
| Need for respiratory support | 59 (32.2%) | 52 (55.9%) | 7 (7.8%) | <0.001 |
| Duration of hospitalization (days) | 8 (6-11) | 8.5 (6-11) | 8 (5-10) | 0.044 |
| Time to swab negativity (days) | 8 (5-11) | 8 (6-11) | 7 (5-11) | 0.071 |
| Mortality | 24 (13.1%) | 19 (20.4%) | 5 (5.6%) | 0.003 |
COPD=chronic obstructive pulmonary disease; COVID-19=coronavirus disease 2019; HIV=human immunodeficiency virus; NLR=neutrophil-lymphocyte ratio; CRP=C-reactive protein; LDH=lactate dehydrogenase; HD=hemodialysis. Data are presented as n (%), mean±SD, or median (interquartile range). Statistically significant results are in bold
Characteristics associated with symptomatic presentations
Patients who were asymptomatic at the time of diagnosis, and identified by routine screening at their respective units, accounted for 50.8% of our cohort. Among the 93 patients who were symptomatic at presentation, fever, cough, and breathlessness were present in 81 (87.1%), 63 (67.7%), and 59 (63.4%) patients, respectively. There was no significant difference between age, sex, coexisting conditions, dialysis access, or dialysis vintage among patients who were symptomatic versus asymptomatic at presentation [Table 1]. However, the inflammatory markers of C-reactive protein (CRP), lactate dehydrogenase (LDH), and ferritin were significantly higher among those who were symptomatic at presentation (P < 0.001, 0.011, and 0.003, respectively). Similarly, higher CT grades were significantly associated with patients who had symptoms at presentation (P < 0.001). These patients were also more likely to require respiratory support (P < 0.001) and had significantly higher mortality rate (P = 0.003).
Factors associated with mortality
In a univariate analysis [Table 2], the factors that were independently associated with mortality included age ≥60 years (odds ratio [OR], 9.357; 95% confidence interval [CI] [3.649-23.995]; P < 0.001), having symptoms at presentation (OR, 4.365; 95% CI [1.553–12.266]; P = 0.004), neutrophil–lymphocyte ratio (NLR) >6 (OR, 3.401; 95% CI [1.414–8.184]; P = 0.004), CRP >20 mg/L (OR, 8.875; 95% CI [2.018–39.023]; P = 0.001), serum LDH >250 IU/L (OR, 4.968; 95% CI [1.423–17.342]; P = 0.006), CT Grades 3 and 4 (OR, 4.767; 95% CI [1.751–12.978]; P = 0.001), and need for respiratory support (OR, 5.395; 95% CI [2.154–13.511]; P < 0.001). A multivariate logistic regression analysis was performed [Table 3], including all variables that were found to be predictors of mortality on univariate analysis. The only factor that retained significance was age ≥60 years (OR, 21.501; 95% CI [2.389–193.485]; P = 0.006).
Table 2.
Univariate analysis on the association between mortality and various demographic, clinical, laboratory, and radiological characteristics
| Variables | Died (n=24) | Survived (n=159) | P | OR (95% CI) |
|---|---|---|---|---|
| Age ≤39 years | 2 (8.3%) | 38 (23.9%) | 0.085 | 0.289 (0.065-1.288) |
| Age 40-59 years | 6 (25.0%) | 93 (58.5%) | 0.002 | 0.237 (0.089-0.628) |
| Age ≥60 years | 16 (66.7%) | 28 (17.6%) | <0.001 | 9.357 (3.649-23.995) |
| Male sex | 19 (79.2%) | 99 (62.3%) | 0.107 | 2.303 (0.817-6.490) |
| Symptomatic at presentation | 19 (79.2%) | 74 (46.5%) | 0.004 | 4.365 (1.553-12.266) |
| Coexisting conditions | ||||
| Hypertension | 18 (75%) | 122 (76.7%) | 0.852 | 0.910 (0.337-2.460) |
| Diabetes Mellitus | 11 (45.8%) | 51 (32.1%) | 0.247 | 1.792 (0.751-4.274) |
| Heart failure | 5 (20.8%) | 40 (25.5%) | 0.647 | 0.783 (0.274-2.233) |
| COPD/asthma | 1 (4.2%) | 10 (6.3%) | 1.000 | 0.648 (0.079-5.301) |
| Laboratory features | ||||
| NLR >6 | 13 (54.2%) | 41 (25.8%) | 0.004 | 3.401 (1.414-8.184) |
| CRP >20 mg/L | 22 (91.7%) | 88 (55.3%) | 0.001 | 8.875 (2.018-39.023) |
| LDH >250 IU/L | 21 (87.5%) | 93 (58.5%) | 0.006 | 4.968 (1.423-17.342) |
| Ferritin >500 ng/mL | 20 (83.3%) | 122 (76.7%) | 0.470 | 1.516 (0.488-4.717) |
| Imaging findings | ||||
| CT not suggestive of COVID-19 | 6 (26.1%) | 75 (47.2%) | 0.057 | 0.395 (0.148-1.055) |
| CT Grade 1 or 2 | 9 (39.1%) | 68 (42.8%) | 0.741 | 0.860 (0.352-2.104) |
| CT Grade 3 or 4 | 8 (34.8%) | 16 (10.1%) | 0.001 | 4.767 (1.751-12.978) |
| Renal failure details | ||||
| Permanent access | 15 (62.5%) | 124 (78%) | 0.098 | 0.470 (0.190-1.166) |
| Temporary HD catheter | 9 (37.5%) | 34 (21.4%) | 0.083 | 2.206 (0.889-5.476) |
| Dialysis vintage (months) | 21 (1-36) | 18 (6-36) | 0.492 | 1.007 (0.987-1.027) |
| Need for respiratory support | 16 (66.7%) | 43 (27%) | <0.001 | 5.395 (2.154-13.511) |
COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase; COVID-19=coronavirus disease 2019; HD=hemodialysis; CT=computed tomography; OR=odds ratio; CI=confidence interval. Data are presented as n (%), mean±SD, or median (interquartile range). Statistically significant results are in bold
Table 3.
Multivariate logistic regression analysis of the association between mortality and various characteristics
| Variables | OR (95% CI) | P |
|---|---|---|
| Age 40-59 years | 2.038 (0.217-19.125) | 0.533 |
| Age ≥60 years | 21.501 (2.389-193.485) | 0.006 |
| Symptomatic at presentation | 3.125 (0.778-12.560) | 0.108 |
| NLR >6 | 2.704 (0.883-8.281) | 0.081 |
| CRP >20 mg/L | 1.649 (0.294-9.246) | 0.570 |
| LDH >250 IU/L | 4.674 (0.964-22.656) | 0.056 |
| CT Grade 3 or 4 | 1.437 (0.370-5.578) | 0.600 |
| Need for respiratory support | 3.058 (0.815-11.467) | 0.097 |
NLR=neutrophil-lymphocyte ratio; CRP=C-reactive protein; LDH=lactate dehydrogenase; CT=computed tomography; OR=odds ratio; CI=confidence interval
Analysis of cause of death
Twenty-four patients (13.1%) died either during initial hospitalization or within 2 weeks after discharge [Figure 1]. The most common causes of death included encephalopathy with no identifiable cause (n = 6), respiratory failure (n = 5), and septic shock (n = 4). One patient developed a pseudo-aneurysm of his AV (arteriovenous) fistula, the rupture of which resulted in hemorrhagic shock despite surgical ligation being attempted. The cause of death for two patients could not be ascertained as they died after discharge.
Figure 1.
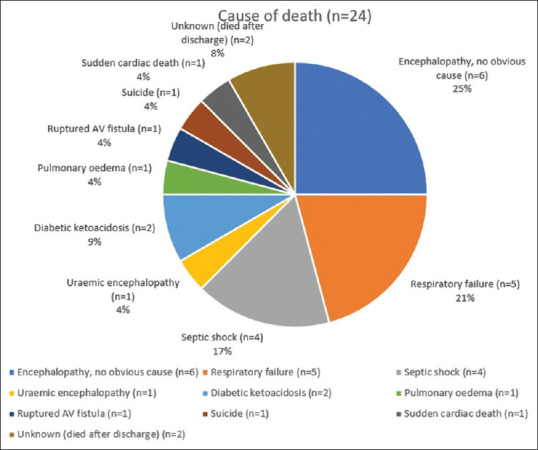
Cause of death analysis
Discussion
Several months have now passed since the first cases of COVID-19 were reported in December 2019. However, there remains a paucity of data on the effects of the disease on patients with ESKD who are on maintenance hemodialysis. Several case series have been published from different centers around the world [Table 4], the largest so far being from London, UK, with 300 patients.[11] Our study provides one of the first Indian perspectives on the clinical course and outcomes of COVID-19. Patients with ESKD on dialysis are at an increased risk for contracting COVID-19 because of the need for frequent contact with health care environments, close physical proximity with other patients during the dialysis session, the underlying immune dysregulation associated with CKD, and the frequent presence of comorbid conditions such as diabetes mellitus, hypertension, and cardiovascular disease.[15]
Table 4.
Comparison of our cohort with other published case series of end-stage kidney disease patients with COVID-19
| Country | Wuhan, China | Brescia, Italy | Madrid, Spain | New York, USA | London, UK | Paris, France | Chennai, India |
|---|---|---|---|---|---|---|---|
| Author | Xiong et al.[12] | Alberici et al.[15] | Goicoechea et al.[6] | Valeri et al.[13] | Corbett et al.[11] | Tortonese et al.[14] | [Current paper] |
| Number of patients (n) | 131 | 94 | 36 | 59 | 300 | 44 | 183 |
| Age (mean/median) | 63.3 | 72 | 71 | 63 | 66 | 61 | 49.97 |
| Asymptomatic cases (%) | 21.4% | 19.% | - | 5% | - | - | 50.8% |
| Diabetes mellitus (%) | 22.9% | 43% | 64% | 69% | 51.7% | 50% | 33.9% |
| Hypertension (%) | 68.7% | 93% | 97% | 98% | - | 97.7% | 76.5% |
| Mortality (%) | - | 25.5% | 30.6% | 31% | 20.3% | 27.3% | 13.1% |
COVID-19=coronavirus disease 2019
However, about half of our patients were completely asymptomatic at presentation and were identified only through screening protocols at their dialysis centers. The proportion of patients who presented asymptomatically was much higher compared with other case series Table 4, and their presence underscores the need for increased vigilance within dialysis centers, to avoid cross-infection to both patients and dialysis staff.
Our cohort, like previously published reports,[5,6,7] failed to identify an association between mortality and comorbid conditions such as diabetes mellitus or hypertension. However, it has become increasingly apparent that COVID-19 has a unique predilection to worsen glycemic control in diabetics, and even cause hyperglycemia in previously nondiabetic individuals; both have been linked with poor clinical outcomes.[16] This propensity to hyperglycemia was noticed in our patients as well, with diabetic ketoacidosis even resulting in the death of two of our patients.
Biochemical evidence of systemic inflammation in the form of raised CRP and LDH, along with an elevated NLR, were all found to be independent predictors of mortality. Serum ferritin, however, was not – potentially because of the confounding effect of repeated IV iron infusions in this patient population. The use of IV iron and erythropoiesis-stimulating agents was halted for all our patients, irrespective of hemoglobin levels, as iron therapy is contraindicated during active infection, and erythropoietin could potentially worsen the reported hypercoagulability associated with COVID-19, although no such events were noted in our patient population during the study period.
Imaging abnormalities on chest CT were detected in 43.8% of patients who were asymptomatic, highlighting its utility as a potential screening tool as suggested by Xiong et al.[12] However, in the ESKD population, the findings of pulmonary congestion sometimes overlap with the ground glass opacities of COVID-19, complicating their interpretation. CT findings more in favor of pulmonary edema, versus COVID-19 pneumonia, included pleural effusions, cardiomegaly, and central distribution.[17] Patients with high-grade CT findings (>50% of lung involvement) are noted to have an increased risk of mortality. Wide variations were noted in the time taken for patients to become negative for SARS-CoV-2 by nasopharyngeal swab RT-PCR. The median duration was 8 days, although one patient remained positive for a total of 24 days.
The overall mortality in our analysis was 13.1%, which is higher than that reported in the general population (1.4%–8%[6]). Nevertheless, it remains lower than that reported in the published literature for ESKD patients [Table 4]. One potential contributor might have been our early adoption of steroid therapy, which had become common practice well before the results of the steroid arm of the RECOVERY (Randomized Evaluation of COVID-19 Therapy) trial were known, in accordance with the local guidelines.[9] Other possible factors that might have contributed to the lower mortality rate include the lower mean age compared with other studies [Table 4], the policy of universal admission irrespective of symptomatology, permitting closer monitoring and early intervention in case of clinical deterioration, and the policy of routine CT imaging, which might have assisted risk stratification. However, these potential explanations remain hypothetical.
Strengths of the study
Our study represents the largest Indian cohort of ESKD patients with COVID-19 published so far. Being a large tertiary-care center, referrals to our institution have come from more than 64 different dialysis units, resulting in a wide representation across different geographical locations. The separation of patients into symptomatic and asymptomatic groups was based on self-reported symptoms at the time of testing for COVID-19. Since most patients, on presentation, had missed their last scheduled dialysis session on account of having tested positive, this methodology avoided the confounding effect of pulmonary congestion (whose symptoms of dyspnea and cough overlap with those of COVID-19) on the analysis of symptomatology. However, some patients who were asymptomatic at presentation did go on to develop symptoms.
Limitations of the study
Only patients who were given at least one session of hemodialysis at our center were included in the analysis; therefore, some amount of selection bias may have occurred, whereby patients who were too ill for hemodialysis or who died before hemodialysis could be initiated were not included. The wide confidence intervals for some of the analyses suggest that larger studies may yet be required.
Conclusion
This analysis confirms the previous reports of higher COVID-19-related mortality in the dialysis population vis-à-vis the general population and identifies older age, higher inflammatory markers, and greater degrees of radiological lung involvement to correlate with increased mortality. Further studies, however, will be required to more clearly elucidate the natural history of the disease in the dialysis population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. WHO Director-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV) [Last accessed on 2020 Aug 03]. Available from: https://wwwwhoint/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov)
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:P653–61. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–6. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, Rojas ÁG, et al. COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana F, Giaroni F, Frisina M, Alfano G, Mori G, Lucchi L, et al. SARS-CoV-2 infection in dialysis patients in northern Italy: A single-centre experience. Clin Kidney J. 2020;13:334–9. doi: 10.1093/ckj/sfaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.State Control Room, Directorate of Public Health and Preventive Medicine, Health and Family Welfare Department, Government of Tamil Nadu. Media Bulletin. [Last accessed 2020 Aug 03]. 01072020 Available from: https://stopcoronatngovin/wp-content/uploads/2020/07/Media-Bulletin-01072020-23-Pages-English-445-KBpdf .
- 9.Government of India, Ministry of Health and Family Welfare, Directorate General of Health Services (EMR Division) Guidelines on Clinical Management of COVID-19. [Last accessed on 2021 April 30]. Availablefrom: https://wwwmohfwgovin/pdf/RevisedNational ClinicalManagementGuidelineforCOVID1931032020pdf .
- 10.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19-Preliminary Report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, et al. Epidemiology of COVID-19 in an Urban Dialysis Center. J Am Soc Nephrol. 2020;31:1815–23. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387–97. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–15. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortonese S, Scriabine I, Anjou L, Loens C, Michon A, Benabdelhak M, et al. COVID-19 in patients on maintenance dialysis in the Paris region. Kidney Int Rep. 2020;5:1535–44. doi: 10.1016/j.ekir.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikizler TA. COVID-19 and dialysis units: What do we know now and what should we do.? Am J Kidney Dis. 2020;76:1–3. doi: 10.1053/j.ajkd.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. 2020;163:108186. doi: 10.1016/j.diabres.2020.108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino M, Itoh H, Hatabu H. A practical approach to high-resolution CT of diffuse lung disease. Eur J Radiol. 2014;83:6–19. doi: 10.1016/j.ejrad.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


