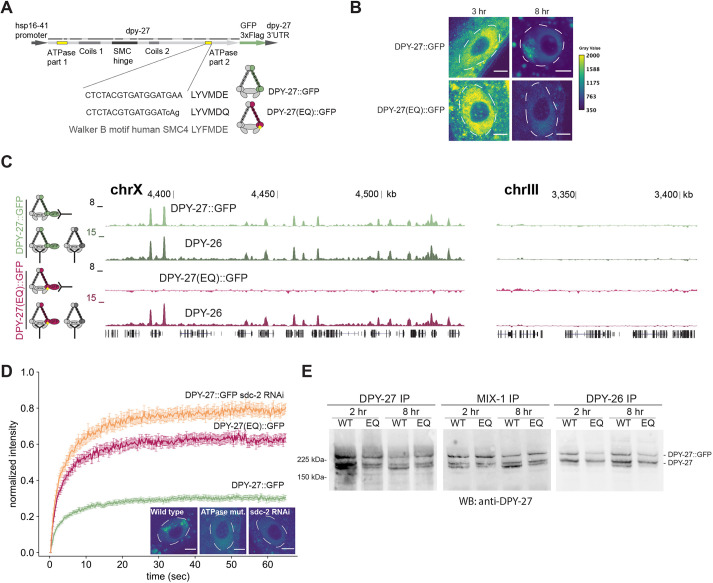
Fig. 2.
The effect of a conserved SMC ATPase mutation on DPY-27 binding, function, protein stability and complex formation. (A) Sequence encoding heat shock-inducible GFP-tagged DPY-27(EQ). The DNA sequence coding for the conserved Walker B motif and the E-to-Q mutation are shown below. (B) Localization of the wild-type and EQ ATPase mutant DPY-27::GFP proteins in intestine cells. Adults were heat shocked at 35°C for 1 h and recovered for either 3 h or 8 h. Unlike DPY-27::GFP, the ATPase EQ mutant did not show subnuclear localization. Nuclei are outlined by white dashed lines. Images are representative of three replicates (quantified in Fig. S3E). Scale bars: 5 µm. (C) ChIP-seq analysis of wild-type and ATPase mutant DPY-27::GFP using an anti-GFP antibody in embryos. ChIP against DPY-26 was used as a positive control in the same extracts. Unlike the wild-type protein, the ATPase mutant failed to bind to the X chromosome (chrX), and both did not localize to the autosomes. A representative region from chromosome III is shown in the right panel. ChIP profiles show normalized read coverage (y-axis) for representative regions on chromosome X and III in a UCSC genome browser snapshot. Data are representative of three replicate experiments. (D) Mean FRAP recovery curves from DPY-27::GFP, DPY-27(EQ)::GFP and DPY-27::GFP upon SDC-2 RNAi. FRAP was performed ∼8 h after the heat shock. Data are mean±s.e.m. Numbers of bleached single intestine nuclei (from at least three biological replicates) for each experiment are n=81 for DPY-27::GFP, n=37 for DPY-27(EQ)::GFP and n=32 for DPY-27::GFP sdc-2 RNAi. The images depict examples of intestine nuclei used for FRAP analysis. Unlike DPY-27::GFP, the ATPase EQ mutant did not show subnuclear localization, similar to when condensin DC recruiter SDC-2 was knocked down. Nuclei are outlined by white dashed lines. Scale bars: 5 µm. (E) Co-immunoprecipitation analysis of condensin DC subunits. Protein extracts were prepared from larvae that were heat shocked for 1 h at 35°C and recovered at 20°C for 2 h or 8 h. Immunoprecipitation (IP) of condensin DC subunits DPY-27, DPY-26 and MIX-1 was performed, and immunoprecipitated DPY-27::GFP and endogenous protein were analyzed by western blotting (WB) with an anti-DPY-27 antibody. The intensity of the DPY-27::GFP and endogenous protein bands in the DPY-27 IP lane indicates the relative abundance of each protein. The intensity of DPY-27::GFP and endogenous protein bands in other lanes indicates their relative interaction with each subunit. Blots are representative of two experiments.