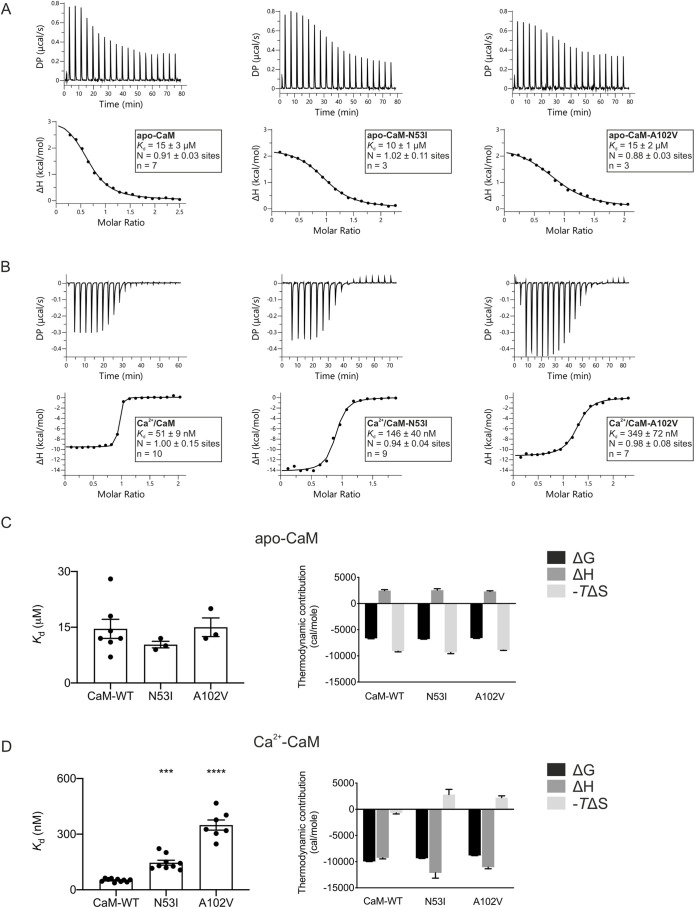
Fig. 1.
Ca2+/CaM binding to RyR2 is decreased for the CPVT-associated variants N53I and A102V. (A,B) Representative ITC titration curves (upper panels) and binding isotherms (lower panels) for CaM interaction with RyR23583-3603 in the absence (A) and presence (B) of Ca2+. DP, differential power. (C,D) Affinity (left) and thermodynamic profile (right) of the binding of apo-CaM (C) and Ca2+/CaM proteins (D) to RyR23583-3603 obtained by fitting to a one-site binding model. Data were processed using the MicroCal PEAQ-ITC software. Data are mean±s.e.m. N, stoichiometry; n, number of experimental replicates. The sum of the change in enthalpy (ΔH) and the change in entropy (ΔS) multiplied by the absolute temperature (T) gives the change in free energy (ΔG). Experiments were performed in the presence of 5 mM EGTA or 5 mM CaCl2 at 25°C. Number of replicates (n) for each condition is shown in the lower panels of A and B. ***P<0.001; ****P<0.0001 (versus CaM-WT; differences between three groups were determined using one-way ANOVA with Dunnett's post-hoc test).