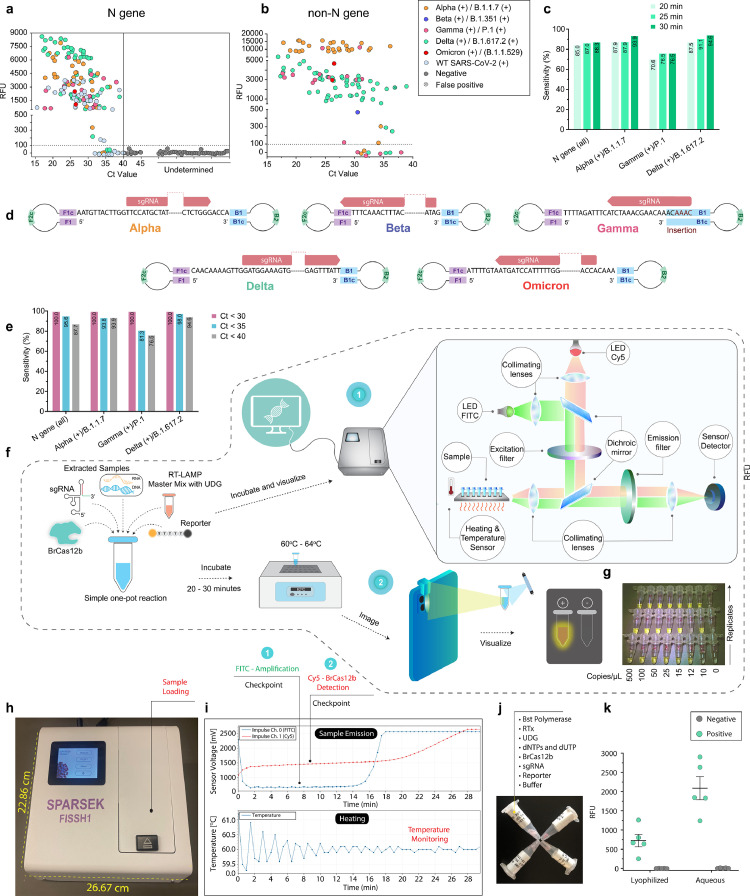
Figure 2.
Clinical validation of one-pot BrCas12b detection assay and portable diagnostic systems. (a) and (b) One-pot patient sample detection. The N gene target indicates the presence of SARS-CoV-2 while non-N gene targets indicate detection of variants. Fluorescence measurements were taken at t = 30 min. (c) Percent accuracy of the one-pot detection at 20-, 25-, and 30 min targeting variants and WT SARS-CoV-2. (d) Self-hybridising loop structure acts as a seed for exponential LAMP amplification which subsequently serves as a basis for BrCas12b one-pot detection of VOCs. Guide RNA designed to target the dumbbell region of the LAMP products are shown. (e) Percent accuracy with respect to Ct value for SARS-CoV-2 variant detection. (f) One-pot assembly and comparison of a portable in-house detection instrument (FISSH) and an at-home detection method using a mobile phone with a simple lens. (g) Depiction of image taken by the mobile phone in (e). (h) and (i) FISSH footprint and dual-channel graph generated from a positive sample. (j) Representation of lyophilised one-pot detection reaction. (k) Lyophilised samples compared to the standard one-pot reaction. Fluorescence measurements were taken at t = 30 min and the Mean±SD (n = 5 technical replicates) are indicated.