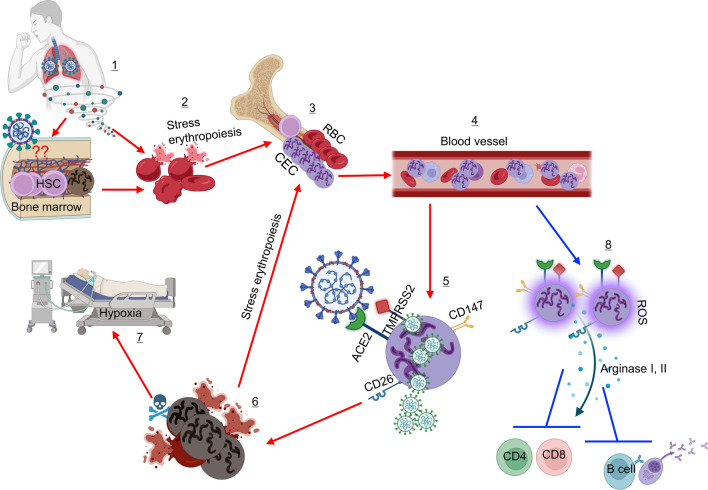
Fig. 3.
This model illustrates the impact of SARS-CoV-2 infection on hematopoiesis. Stress erythropoiesis characterized by the expansion of erythroid precursors/progenitors (CECs) in the peripheral blood of COVID-19 patients might be the result of direct invasion of HSCs in the bone marrow (1). In this scenario, erythroid progenitors get infected/lysed (2) and the bone marrow as a compensatory mechanism generates more CECs (3). Some of these CECs egress the bone marrow before maturing to RBCs and entering the blood circulation (4). This might be one potential reason for the massive number of CECs in the blood circulation of COVID-19 patients, especially in those with a severe disease. Alternatively, CECs may get exposed to the virus in damaged tissues of the lungs. It is reported that CECs are prone to infection (5) as they express the required receptors for SARS-CoV-2 (e.g. ACE2, TMPRSS2, CD147, and CD26). Therefore, infection of CECs results in their elimination (6), which results in a vicious cycle of stress erythropoiesis (3) and at the same time hypoxia in COVID-19 patients (7). In addition, expanded CECs via the secretion of arginase I, II, and ROS can suppress the proliferation and effector functions of T and B cells (8). Besides, there are multiple reports showing structural alterations/damages of mature RBCs in COVID-19 patients