Abstract
In 1908, Bleuler proposed a unitary theory of schizophrenia, hypothesizing a “loosening of associations” as the central mechanism underlying disturbances in thinking, motivation, and affective expression. Here, we test Bleuler’s model in an archival sample of 79 healthy controls and 76 patients with chronic schizophrenia who had completed neuropsychological tests, including a measure of learning of novel word pairs, which was specifically selected to probe the structure and formation of new verbal associations. The patients also had positive and negative symptoms ratings, including measures of flat affect, anhedonia, and thought disorder. A subset of patients and controls (n = 39) had available prior archival 3-T magnetic resonance imaging (MRI) measures of prefrontal cortex (PFC) gray matter volumes. In relation to controls, patients showed evidence of a selective impairment in associative learning, independent of their overall reduced neuropsychological functioning. This neuropsychological impairment, in turn, correlated significantly with overall levels of negative but not positive symptoms, with the data showing an especially strong contribution of flattened emotional expression to verbal associate learning deficits in this patient sample. Moreover, the archival MRI data were consistent with prior research pointing to an important role of the PFC in supporting verbal associate learning and memory in patients and controls. Taken together, the current results provided evidence of a selective impairment in schizophrenia on a PFC-supported verbal associate learning and memory task, which was accompanied by negative symptoms in general, and flattened emotional expression, in particular.
Keywords: schizophrenia, Bleuler, associative learning, negative symptoms, prefrontal cortex
Introduction
Bleuler first coined the term “schizophrenia” in 1908 to denote the “splitting” of different mental domains that he viewed as the central and unifying mechanism underlying the broad, varied, and complex psychological disturbance that defined the illness.1,2 In choosing the name schizophrenia, which literally means “a mind that is torn asunder,” Bleuler placed center stage disruption in the efficient and economical coordination of mental processes, with a so-called loosening of associations resulting in a fragmented phrene or mind.3 He viewed this cognitive disturbance in associative threads that bind and sequence word, ideas, and thoughts into logical discourse as occurring against a backdrop of distinct and notable reductions in emotional expression and motivated, goal-directed decision making, described as flattened affective expression and ambivalence, respectively.4
In support of Bleuler’s model, early behavioral studies reported deficits in associate learning on laboratory verbal recall tasks in both remitted and non-remitted schizophrenia,5 which later investigations showed to be distinct from disease-related disturbances in other aspects of memory.6 Subsequent meta-analytic evidence7 pointed to specific difficulties for patients with schizophrenia in the associative aspects of memory that are essential for binding elements of an episode into cohesive, to-be-remembered percepts.8,9 Ragland et al,10 for example, compared the effects of item-specific versus relational encoding on memory performance in schizophrenia. During the encoding/study phase of the experiment, participants made judgments as to whether the target stimulus was either living/nonliving (item-specific) or belong together (relational). Results indicated that patients performed much worse when instructed to process relationships between items during encoding instead of focusing on individual item features.10 As Ragland and colleagues noted, relational processing promotes encoding of associations that subsequently aids in recognition accuracy for controls but not patients.10
Findings from structural and functional brain imaging studies have also offered strong support for a selective impairment in associative learning and memory in schizophrenia. Maher et al,11 for example, investigated the effects of context on verbal memory performance in patients who had undergone structural magnetic resonance imaging (MRI) studies of frontal gray matter volumes. They reported that frontal gray matter volumes correlated with verbal memory for tasks that placed heavy demands on contextual organization of to-be-remembered material. By contrast, recall of simple, single-item word lists did not correlate with frontal gray matter volumes in this sample of patients with schizophrenia.11 Similarly, in a functional MRI study, Oertel et al12 showed, in comparison to both unaffected first-degree relatives and controls, patients had reduced associative learning and memory of face–name pairs which in turn correlated with abnormal patterns of activation predominantly in the default mode network, including prefrontal cortex (PFC), middle frontal gyrus, hippocampus, and inferior parietal lobe.12
Taken together, these contemporary cognitive neuroscience studies help to identify abnormalities in informational and neural structures and functions that Bleuler long presumed to underlie associative disturbance in schizophrenia. That these studies all involved behavioral tasks that place heavy demands on learning, such as word lists or face–name pairs has taken on increasing importance for several theoretical and clinical reasons. First are recent meta-analytic findings pointing to medium-to-large deficits on similar verbal tests of learning in individuals at clinical high risk for psychosis.13 Second is the growing body of evidence linking poor associative learning with impaired N-methyl-d-aspartate-type glutamate receptor (NMDAR)-mediated synaptic plasticity in schizophrenia,14 with a recent meta-analysis of post mortem brain studies revealing that such abnormalities in synaptic pruning are overrepresented in temporal and frontal cortical regions (see Berdenis van Berlekom et al15). From this perspective, schizophrenia compromises NMDAR signaling essential for experience-dependent plasticity, a fundamental property of the brain that allows it to change in response to external input, learning, and training.14 Of particular relevance are data showing how these long-term potentiated (LTP) and long-term depressed (LTD) plastic changes may help to form and unite distinct networks of brain regions that are thought to underlie selective forms of learning and cognition. In fact, NMDAR signaling via administration of d-cycloserine (DCS) has been shown to have a selective effect on specific cognitive tasks, improving associative learning but not working memory in healthy participants.16
A key research question is how these contemporary cognitive neuroscientific findings might be understood in relation to the loosening of associations that Bleuler long viewed as central to both the pathophysiology and clinical expression of schizophrenia. For example, does Bleuler’s loosening of associations manifest as a unified and distinct pattern across cognitive, symptom, and structural brain imaging measures? To address these questions, the current study employed a multifactorial within-subjects design aimed to interrogate Bleuler’s loosening of association construct across neuropsychological, symptom, and structural MRI measures. For neuropsychological measures, the study included the Verbal Paired Associates (VPA) task, a subtest of the Wechsler Memory Scale, third edition (WMS-III)17 and the Working Memory Index (WMI) of the Wechsler Adult Intelligence Scale, third edition (WAIS-III). Crucially, these 2 tasks allowed for within-subjects comparisons of 2 related but distinct aspects of cognitive functioning—associative learning and working memory. The VPA provided a measure of learning, tracking performance incrementally across 4 study trials in which subjects are read a list of 8-word pairs (eg, truck-bag) followed by a cued recall test (eg, what word went with truck?), with the correct answer provided, if necessary (eg, bag went with truck). By comparison, the working memory tasks used in this study emphasized attentional control and arithmetic computations but did not track learning, as assessed by changes in performance with study and practice.18 As noted, recent neuropharmacological research highlights the distinction between learning tasks and working memory exercises with NMDAR signaling via administration of DCS showing a selective effect on specific cognitive tasks, improving incremental or associative learning but not working memory in healthy participants.16
We now present evidence bearing on the pivotal research question as to whether: (a) Bleuler’s hypothesized failure in associate learning in schizophrenia can be distinguished from other notable disease-related cognitive impairments, particularly those related to disturbances in working memory and (b) whether it is related to specific symptoms of flat affect, reduced motivation and thought disturbance that Bleuler so long ago emphasized. In addition, for a subset of patients and controls, we had available archival data of MRI gray matter volumes of the PFC along with VPA performance.19 Findings of several brain imaging studies show that performance on neuropsychological measures of learning and memory, such as the VPA involve episodic encoding and retrieval processes supported by brain pathways connecting PFC and medial temporal lobe, particularly hippocampus structures (eg, Allen et al,20 Anderson et al,21 Dolan and Fletcher,22 and Nee and Jonides23). We therefore examine VPA performance in relation to PFC gray matter volume in this subset of patient and controls. However, because the MRI data of this subset of patients and controls were not collected at the same time of the neuropsychological evaluations, testing of brain–behavior correlations is clearly limited. Indeed, the estimated time interval of 24–36 months between cognitive and MRI evaluations did not allow for testing of a specific PFC hypothesis of VPA impairment in schizophrenia. Nevertheless, these data may help to address whether the broader relationship widely reported in the literature linking PFC with learning and memory (eg, Allen et al,20 Anderson et al,21 Dolan and Fletcher,22 and Nee and Jonides23) is evidenced in the current study.
Methods
Participants
The archival sample consisted of research participants (N = 155) between the ages of 17 and 55 years, right-handed, native speakers of English, without histories of electroconvulsive therapy (ECT), neurological illness, and without alcohol or drug abuse in the past 5 years. All research participants gave informed consent prior to their participation in the study. Diagnoses for patient group were ascertained by the Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-P)24 along with chart review. All patients (n = 76) had been part of a comprehensive, longitudinal study of schizophrenia, and all were receiving neuroleptic medication; the mean chlorpromazine (CPZ) equivalent daily dose was 482.31.50 mg (SD = 403.56).25 Patients had a mean age of 40.38 years (SD = 9.94), mean years of education of 12.90 years (SD = 2.17), and a mean duration of illness of 17.70 years (SD = 10.41). Control participants (n = 79), recruited from newspaper and online advertisement, underwent the Structured Clinical Interview for DSM-IV Axis I Disorders—Non-patient Edition (SCID-NP), had a mean age of 41.43 years (SD = 8.74), mean years of education of 15.23 years (SD = 8.74), and were equated to patients based on age and handedness. Seventy-six (12 females) patients and 79 (26 females) healthy controls completed the WMS-III,17 and the WAIS-III.26 Of these participants, 25 male patients and 14 male controls also had available prior 3-T MRI studies which we have previously reported, showing gray matter volume reductions in specific PFC regions in patients relative to controls.19
Measures
The WAIS-III yielded composite measures of intelligence (Full-Scale IQ, Verbal IQ, Performance IQ) and index scores of Verbal Comprehension, Perceptual Organization, Working Memory, and Processing Speed26 (Wechsler, 1997). The WMS-III provided the following index scores: Auditory Immediate Memory, Visual Immediate Memory, Immediate Memory, Auditory Delayed Memory, Visual Delayed Memory, and General Delayed Memory.17
Participants completed the VPA subtest of the WMS-III. For the VPA subtest, participants are asked to learn 8, orally presented unrelated word pairs (eg, truck-bag) across 4 study test trials. The VPA yielded 4 raw scores (number of word pairs correctly remembered for each of the 4 study trials), summed and converted to an immediate recall scale score (VPA I) and a delayed recall score (number of words pairs correctly remembered following a 30-min delay) (VPA II).17 These scores provided a quantitative measure of associate learning and episodic memory by testing immediate recall of specific word pairs after each of the 4 study trials (eg, what word went with truck?), and delayed recall (eg, what word went with truck?) following a 30-minute interval.17
The Scale for the Assessment of Negative Symptoms (SANS)27 and the Scale for Assessment of Positive Symptoms (SAPS)28 are clinical assessment instruments that are widely used to rate negative and positive symptoms in schizophrenia. The SANS consists of 19 items representing 5 rationally derived subscales: Affective Flattening or Blunting, Alogia, Avolition–Apathy, Anhedonia–Asociality, and Inattention. The SAPS consists of 30 items representing 4 rationally derived subscales: Hallucinations, Delusions, Bizarre Behavior, and Positive Formal Thought Disorder. Each subscale yields a score, a Likert rating of severity ranging from 0 = none, 1 = questionable, 2 = mild, 3 = moderate, 4 = marked, to 5 = severe. Empirical studies have shown that negative symptoms can be reliably distinguished from positive symptoms.29 However, studies using factor analysis have pointed to a much more complex, multidimensional symptom structure, with 3- or 5-factor models marshalling the most empirical support.29,30 Accordingly, we used the 5 SANS scores and the 4 SAPS scores to rate symptoms in schizophrenia.
MRI Processing
As outlined by Ohtani et al,19 MR images were acquired with a 3-Tesla General Electric scanner (GE Medical Systems, Milwaukee, WI) at the Brigham and Women’s Hospital in Boston, Massachusetts. A 3-dimensional Fourier transformed spoiled gradient-recalled (fast SPGR) acquisition sequence yielded a coronal series of contiguous 1.0 mm images (TE = 3 ms, TR = 7.4 ms, TI = 600, 10° flip angle, field of view = 25.6 × 25.6 cm, acquisition matrix = 256 × 256, voxel dimension = 1 × 1 × 1 mm). Next, a XETA (eXtended Echo Train Acquisition) sequence yielded a series of contiguous axial T2-weighted images (TE = 80 ms, TR = 2500 ms, field of view = 25.6 × 25.6 cm, voxel dimensions = 1 × 1 × 1 mm). The T2 images from the XETA sequence were registered to the SPGR images. An expectation–maximization (EM) segmentation technique was used to segment the images into 3 major tissue classes: gray matter, white matter, and cerebrospinal fluid, using both SPGR and T2-weighted MR information as well as spatial priors. Intracranial contents (ICCs) included the volume of all tissue classes. For manual tracing of the PFC region of interest (ROI), images were realigned using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt and resampled into isotropic voxels (1 mm3). This realignment procedure was essential for precise and consistent ROI delineation. Using the segmented gray matter, the PFC ROIs were manually drawn on each coronal slice of the realigned SPGR according to the neuroanatomical definitions described below. Three-dimensional information was used including parasagittal, axial, and coronal views, to provide reliable delineation of the PFC ROI using a software package for medical image analysis (3D slicer, http://www.slicer.org) operating on Linux workstations.
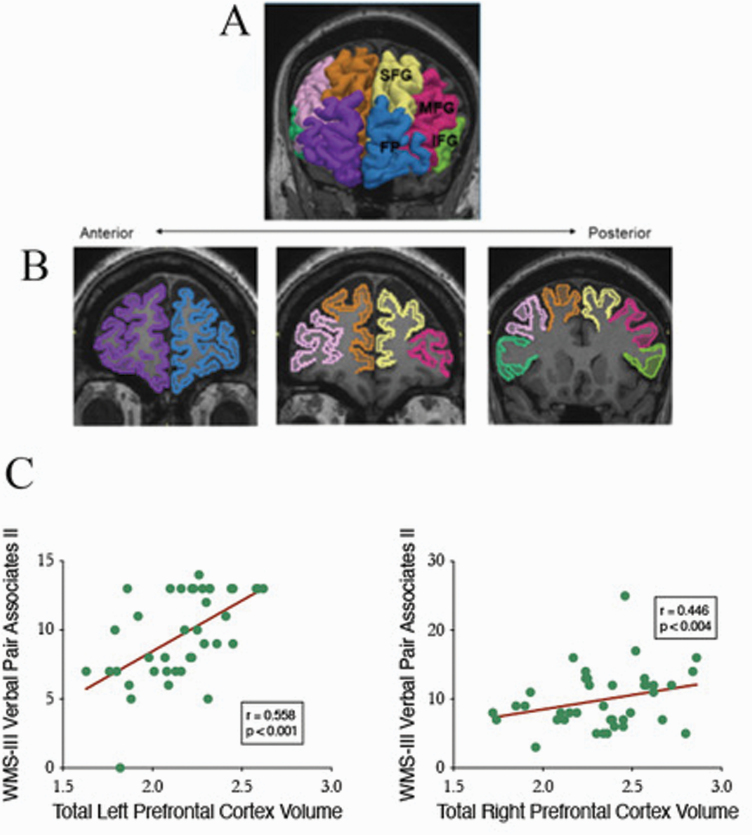
As detailed in Ohtani et al,19 the PFC was parcellated into (a) frontal pole (FP), (b) superior frontal gyrus (SFG), (c) middle frontal gyrus (MFG), and (d) inferior frontal gyrus (IFG). These PFC subregions are displayed in figure 1. The relative volume is calculated as: absolute volume/ICC * 100 (%). All manual delineations were performed by co-author T.O., who was blind to the diagnosis of the subjects. As noted in Ohtani et al,19 to examine interrater reliability, 2 independent raters blind to diagnosis independently delineated bilateral frontal pole, superior frontal gyrus, middle frontal gyrus, and inferior gyrus for 5 randomly selected cases. For 5 randomly selected cases, the intraclass correlation coefficients were 0.98 for the left FP, 0.97 for the right FP, 0.96 for the left SFG, 0.97 for the right SFG, 0.95 for the left MFG, 0.96 for the right MFG, 0.95 for the left IFG, and 0.95 for the right IFG. The neuroanatomical definitions of these subregions can be found in Ohtani et al.19
Fig. 1.
MR images of four prefrontal cortex (PFC) sub-regions. In each case, radiologic convention is followed with left hemisphere of participant shown on viewer’s right. (A) 3-Dimensional reconstruction of four PFC sub-regions. ROIs are superimposed on coronal SPGR image: frontal pole (FP left: blue, right: purple), superior frontal gyrus (SFG left: yellow, right: brown), middle frontal gyrus (MFG left: red, right: pink), and inferior frontal gyrus (IFG left: light green, right: dark green). (B) ROIs in coronal slices of SPGR images. Color coding of (B) is the same of that of (A). Boundary definitions are described in Method. (C) Scatter plots of WMS-III Verbal Paired Associates II and total gray matter volumes for left PFC and right PFC for combined patient and control sample.
Statistical Analyses
Analyses of covariance (ANCOVA) compared patient and control groups on neuropsychological tests of intelligence covarying for education. A series of mixed-models ANCOVAs with one between-subjects factor of group (patient vs control) and one within-subjects factor of index measure tested for both group differences in scores for the WAIS-III and the WMS-III instruments as well for group differences in pattern of index scores for each instrument. In addition, VPA raw scores for study trials 1–4 were submitted to a series of mixed-model ANCOVAs with one between-subjects factor of group (patient vs control) and one within-subjects factor of study trial (study trial: 1–4), controlling for each of 4 WAIS-III indexes—verbal comprehension, perceptual reasoning, working memory, and processing speed. These ANCOVAs provided a direct test of the hypothesis of a selective impairment in verbal associate learning and memory in patients with schizophrenia. In addition, bivariate correlations tested for associations of neuropsychological performance and symptoms for the patient group. Hierarchical regression analyses were then used to quantify the amount of variance in neuropsychological scores that can be specifically accounted for by symptoms of flat affect, anhedonia, and thought disorder. In all regression analyses, the F-to-enter probability was .05, and F-to exclude probability was .10. Last, for the MRI data, bivariate correlations tested for associations of PFC gray matter volumes with neuropsychological test scores including immediate and delayed recall scaled scores for VPA I and VPA II.
Results
Table 1 presents neuropsychological test scores for patients and controls. As shown in table 1, in relation to controls and covarying for the group difference in education levels, patients showed markedly reduced scores across the 4 WAIS-III indexes, F(1,150) = 29.08, P < .001, η p2 = .162, with their performance especially low for processing speed, as reflected by the statistically significant group × index interaction, F(3,450) = 8.15, P = .013, η p2 = .024. Similarly, for the WMS-III, patients showed overall reduced performance across the 4 WMS indexes, which remained significant when covarying for education, F(1,150) = 31.50, P < .001, η p2 = .174.
Table 1.
Neuropsychological Scores for Participants and Symptom Ratings for Patients
| Controls (n = 79) | Patients (n = 76) | |
|---|---|---|
| Demographic information | ||
| Age | 41.43 ± 8.74 | 40.38 ± 9.94 |
| Education | 15.23 ± 8.74 | 13.22 ± 2.03** |
| SES | 2.23 ± 0.97 | 3.47 ± 1.13** |
| WAIS-III IQ | ||
| Full scale | 108.76 ± 12.81 | 88.41 ± 14.68** |
| Verbal | 110.11 ± 12.73 | 90.88 ± 15.57** |
| Performance | 105.58 ± 13.93 | 85.67 ± 13.68** |
| WAIS-III index | ||
| Verbal Comprehension | 108.57 ± 16.05 | 93.68 ± 17.23** |
| Perceptual Organization | 106.02 ± 18.28 | 88.88 ± 16.36** |
| Working Memory | 108.13 ± 17.34 | 87.49 ± 16.06** |
| Processing Speed | 104.31 ± 18.06 | 81.17 ± 15.03** |
| WMS-III | ||
| Immediate | 103.43 ± 14.62 | 81.83 ± 17.19** |
| Auditory Immediate | 104.35 ± 12.73 | 86.65 ± 16.32** |
| Visual Immediate | 100.64 ± 14.85 | 84.29 ± 15.63** |
| General | 105.90 ± 13.96 | 85.93 ± 15.41** |
| Auditory Delayed | 107.37 ± 11.92 | 90.49 ± 17.38** |
| Visual Delayed | 103.79 ± 14.07 | 85.92 ± 16.43** |
| Verbal Paired Associates I | 11.16 ± 3.54 | 8.26 ± 3.04** |
| Verbal Paired Associates II | 11.36 ± 2.44 | 8.58 ± 3.36** |
| SANS/SAPS | ||
| Negative symptoms | N/A | 11.46 ± 6.33 |
| Positive symptoms | N/A | 9.23 ± 3.61 |
Note: Values are means plus or minus SDs. SANS, Scale for the Assessment of Negative Symptoms; SAPS = Scale for the Assessment of Positive Symptoms; SES, socioeconomic status; WAIS-III, Wechsler Adult Intelligence Scale, third edition; WMS-III, Wechsler Memory Scale, third edition.
**P < .01.
We next examined VPA performance for patients and controls covarying for above-documented group differences on WAIS-IV indexes. To examine group differences in associate learning, we submitted raw scores for each of the 4 VPA study trials to a mixed-model analysis of variance (ANOVA) with one between-subjects factor of group (patient vs control) and one within-subjects factor of study trial (study trial: 1–4). ANOVA yielded highly statistically significant effects for group, F(1, 153) = 32.72, P < .001, η p2 = .176 and for the interaction of group × study trial, F(3,459) = 4.25, P = .006, η p2 = .027. In relation to controls, patents showed significantly reduced VPA performance as well as significantly reduced rate of learning across the 4 study trials.
A series of mixed-model ANCOVAs then examined the selectivity of this VPA impairment in relation to other neuropsychological functions in patient and controls. ANCOVA revealed that the main effect for group signifying overall reduced VPA performance for patients remained significant when controlling for Wechsler intelligence indexes of verbal comprehension, F(1,152) = 9.77, P = .002, η p2 = .06, perceptual reasoning, F(1,152) = 9.94, P = .002, η p2 = .06, working memory, F(1,151) = 6.42, P = .012, η p2 = .04, and processing speed, F(1,151) = 9.43, P = .003, η p2 = .059. Similarly, the different rates of learning for patients in comparison to controls, as reflected in the significant group × VPA study test trials (trials 1–4) interaction remained significant when controlling for Wechsler index of perceptual reasoning, F(3,456) = 3.08, P = .027, η p2 = .02 but not for verbal comprehension, working memory, or processing speed. Thus, these ANCOVA results provided support for the hypothesis of a selective impairment in associative learning in memory in schizophrenia that can be distinguished from other aspects of disease-related disturbances in cognitive functioning, as measured by intelligence indexes. In addition, none of the memory measures correlated with daily CPZ equivalent antipsychotic dosage.
Table 2 presents correlations of neuropsychological measures and symptom ratings for the patient sample. Table 2 shows highly statistically significant correlations for total negative but not total positive symptoms across index measures for both WAIS-III and WMS-III (all P’s <.01 except for WAIS-III processing speed, P < .05). Similarly for WMS-III VPA subtest, poor performance on this task of learning and memory correlated with heightened negative symptoms for both immediate (r = −.520, P < .001) and delayed (r = −.323, P = .009) recall scores. Poorer performance for VPA immediate recall correlated very significantly with each of the 5 specific negative symptom subscales: flat affect (r = −.554, P < .001), alogia (r = −.431, P < .001), avolition and apathy (r = −.385, P = .002), social anhedonia (r = −.387, P = .002), and attention (r = −.386, P = .002). By comparison, poorer performance for VPA delayed recall correlated with 3 of the 5 negative symptom subscales: flat affect (r = −.366, P = .003), alogia (r = −.307, P = .014), and attention (r = −.278, P = .025). In addition, partial correlation analyses of total negative symptoms controlling for ratings on the attention subscale revealed a statistically significant association with immediate (pr = −.367, P = .003) but not delayed (pr = −.171, P = .183) VPA performance. In contrast, overall positive symptoms did not correlate with any neuropsychological performance measures, except for the thought disorder subscale which correlated significantly with verbal IQ (r = −.382, P = .001), full scale IQ (r = −.362, P = .002), verbal comprehension (r = −.312, P = .008) as well as auditory immediate memory index (r = −.274, P = .025) and delayed VPA recall subtest (r = −.249, P = .042).
Table 2.
Neuropsychological Scores for Participants and Symptom Ratings for Patients
| SANS | SAPS | |
|---|---|---|
| WAIS-III index | ||
| Verbal Comprehension | −.539** P < .001 |
−.192 P = .132 |
| Perceptual Reasoning | −.426** P < .001 |
−.018 P = .886 |
| Working Memory | −.361** P = .003 |
−.042 P = .741 |
| Processing Speed | −.285* P = .021 |
.092 P = .476 |
| WMS-III index | ||
| Auditory Immediate | −.622** P < .001 |
−.215 P = .09 |
| Visual Immediate | −.300* P = .015 |
.015 P = .910 |
| Auditory Delayed | −.415** P = .001 |
.145 P = .259 |
| Visual Delayed | −.281* P = .023 |
−.023 P = .857 |
| Verbal Paired Associates | ± | ±** |
| Verbal Paired Associates I | −.520** P < .001 |
−.162 P = .206 |
| Verbal Paired Associates II | −.323** P = .023 |
−.184 P = .149 |
Note: Values are Pearson Product Moment correlations. Abbreviations are explained in the first footnote to table 1.
*P < .05; **P < .01.
We next examined the relative contributions of flat affect, anhedonia, and thought disorder to VPA performance in the patient group. First, correlational analyses of the interrelationships among these 3 symptom ratings showed a highly statistically significant association of flat affect with anhedonia (r = .610, P < .001) and, to a lesser extent, with thought disorder (r = .298, P = .021). Thought disorder and anhedonia also correlated significantly (r = .279, P = .028). Second, hierarchical regression analyses showed that when entering all these 3 symptoms simultaneously, only flat affect made a significant contribution to reduced scores for VPA immediate recall (β = −.453, t = 3.14, P = .003). As shown in table 3, for immediate recall, flat affect uniquely accounted for approximately 12.60%–14.98% of the variance in VPA scores, as reflected in values of −.355 and −.387 for semi-partial and partial correlations, respectively. As shown in table 4, for delayed recall, flat affect also contributed significantly when entered with thought disorder (β = −.279, t = −2.15, P = .036), uniquely accounting for 7.07%–7.51% of the variance in performance in VPA scores. By contrast, neither anhedonia nor thought disorder contributed significantly to patients’ reduced scores for immediate nor for delayed VPA recall performance, relative to flat affect.
Table 3.
Summary of Hierarchical Regression Analysis for Flat Affect, Thought Disorder, and Anhedonia With Verbal Paired Associates I (Dependent Variable)
| Model | Variable | B | SE B | β | Zero Order | Partial | Semi-partial |
|---|---|---|---|---|---|---|---|
| 1 | Flat Affect | −1.076 | 0.228 | −.526** | −0.526 | −0.526 | −0.526 |
| R 2 | .277 | ||||||
| F for change in R2 | 22.21** | ||||||
| 2 | Flat Affect | −1.047 | 0.241 | −.512** | −0.526 | −0.489 | −0.499 |
| Thought Disorder | −0.095 | 0.237 | −.047 | −0.200 | −0.53 | −0.045 | |
| R 2 | .279 | ||||||
| F for change in R2 | 0.161 | ||||||
| 3 | Flat Affect | −0.927 | 0.295 | −.453** | −0.526 | −0.387 | −0.355 |
| Thought Disorder | −0.075 | 0.240 | −.037 | −0.200 | −0.042 | 0.035 | |
| Anhedonia | −0.212 | 0.295 | −.103 | −0.387 | −0.095 | −0.081 | |
| R 2 | .285 | ||||||
| F for change in R2 | 0.514 |
*P < .05; **P < .01.
Table 4.
Summary of Hierarchical Regression Analysis for Flat Affect, Thought Disorder, and Anhedonia With Verbal Paired Associates II (Dependent Variable)
| Model | Variable | B | SE B | β | Zero Order | Partial | Semi-partial |
|---|---|---|---|---|---|---|---|
| 1 | Flat Affect | −0.704 | 0.268 | −.326** | −0.326 | −0.326 | −0.326 |
| R 2 | .106 | ||||||
| F for change in R2 | 6.88** | ||||||
| 2 | Flat Affect | −0.602 | 0.280 | −.279* | −0.326 | −0.274 | −0.266 |
| Thought Disorder | −0.336 | 0.276 | −.158 | −0.241 | −0.159 | −0.151 | |
| R 2 | .129 | ||||||
| F for change in R2 | 1.49 | ||||||
| 3 | Flat Affect | −0.592 | 0.344 | −.274 | −0.326 | −0.224 | −0.215 |
| Thought Disorder | −0.334 | 0.280 | −.157 | −0.241 | −0.157 | 0.149 | |
| Anhedonia | −0.018 | 0.344 | −.008 | −0.216 | −0.007 | −0.006 | |
| R 2 | .129 | ||||||
| F for change in R2 | 0.003 |
*P < .05; **P < .01.
Last, a subset of participants had available both MRI studies of PFC (n = 39) and WMS-III VPA scores. For the whole subset, left PFC gray matter volume correlated significantly with higher scores for both immediate (r = .359, P = .025) and delayed (r = .558, P < .001) recall VPA measures. By comparison, right PFC gray matter volume correlated only with higher scores for delayed (r = .446, P = .004) but not immediate (r = .283, P = .08) recall VPA scores. Following Bonferroni correction (based on the 2 VPA measures and 2 MRI regions of left and right PFC gray matter volume), only the correlations of delayed VPA with left and right PFC gray matter volumes remained significant. Figure 1 presents scatter plots of these 2 significant Bonferroni-corrected correlations. When analyzing these correlations for patients (n = 25) and controls (n = 14) separately, none achieved statistical significance, although VPA delayed recall and left PFC gray matter volume approached significance for both patient (r = .379, P = .062) and control (r = .516, P = .059) subgroups. For the WMS-III and WAIS-III index measures, the only statistically significant correlation occurred for WMS-III auditory delayed index with left PFC gray matter volume (r = .325, P = .043) for the whole subset. However, this association did not survive when correcting for multiple correlations based on 8 neuropsychological measures and 2 PFC volumes. Likewise, no Bonferroni-corrected correlation of WMS-III or WAIS-III index measures and PFC gray matter volume emerged for either patient or control subset.
Discussion
The current study investigated Bleuler’s unitary model of schizophrenia with a widely used standardized neuropsychological measure of verbal learning and memory, chosen as a specific probe of cognitive processes hypothesized to underlie loosening of associations. Patients’ performance on this task showed evidence of a selective impairment in learning of verbal word pairs, which was independent of their overall reduced neuropsychological performance across measures of intelligence, processing speed, and working memory. This neuropsychological impairment, in turn, correlated significantly with overall levels of negative but not positive symptoms, with the data showing an especially strong contribution of flattened emotional expression to verbal associate learning deficits in this patient sample. Moreover, MRI data were consistent with prior research pointing to an important role of the PFC in supporting verbal associate learning and memory in patient and controls. Taken together, the current results provided evidence of a selective impairment in schizophrenia on a PFC-supported verbal associate learning and memory task, which was accompanied by negative symptoms in general, and flattened emotional expression, in particular.
In both the clinic and the assessment laboratory, people with schizophrenia show an overall cognitive impairment across a wide range of neuropsychological measures (eg, Gold and Dickinson31). This generalized deficit has long been viewed as the most striking and robust feature of the cognitive phenotype of the illness, which in turn has been empirically supported and validated by structural equation modeling and meta-analytic evidence (eg, Gold and Dickinson,31 Dickinson and Harvey,32 and Dickinson et al33). These studies, which typically rely on behavioral measures, have offered little evidence of differential deficits within a specific neuropsychological domain. More promising results, however, have been reported in studies using multimodal measures, for example combining neuropsychological and structural brain imaging to deconstruct the generalized cognitive deficit in schizophrenia (see eg, Nestor et al34,35). Findings from these studies have suggested that the generalized deficit in schizophrenia may be characterized by a double dissociation of cognition and brain structure, specifically linking reduced diffusion tensor imaging-derived measures of white matter integrity of the uncinate fasciculus with verbal memory deficits and those of the cingulum bundle with faulty executive attention and working memory.34,35
In the current study, the results also demonstrated evidence of generalized or broad cognitive impairment with the patient group showing overall depressed functioning across WAIS-III and WMS-III indexes in relation to controls. However, the pivotal question pertained to the extent to which patients’ performance on a specific task of verbal learning and memory could be disentangled from their generalized deficit. In this regard, the data showed that the patients scored significantly lower than controls on the VPA memory subtest, even when controlling for their Wechsler intelligence indexes of working memory, processing speed, and perceptual reasoning. This suggested that their difficulties on this measurement of verbal learning and associative memory could not be simply or solely attributable to other prominent features of neuropsychological disturbance in schizophrenia, particularly those related to working memory. Such selectivity suggested a disease-related disruption in a specific set of neural-informational processes critical for learning of verbal paired associates yet dissociable from those underlying other aspects of schizophrenia neuropsychological disturbance.
This cognitive dissociation aligns well with recent neuroscientific evidence pointing to distinct cellular machinery and NMDAR signaling underlying tasks of associative or incremental learning versus working memory. For incremental learning tasks such as the VPA, efficient and effective encoding and consolidation of novel stimulus–response contingencies are thought to rely heavily on long-lasting NMDAR signaling for the generation of LTP and LTD synaptic strength distributed widely across cortical networks (eg, Forsyth et al,16 Dolan and Fletcher,22 Arnsten et al,36 and Kreitzer and Malenka37). NMDAR signaling may also mediate short-term representation of stimuli in working memory, but with a distinct set of channel kinetics from those that regulate structural LTP synaptic changes underlying experience-dependent learning.38 Indeed, the cellular dynamics of working memory are thought to be characterized by recurrent excitation of dorsolateral PFC microcircuitry in layer III glutamatergic pyramidal neurons.22,38 In schizophrenia, then, patients’ VPA deficit may point to a disease-related failure in verbal learning that is heavily dependent on LTP-mediated plasticity and distinct from the disruption in reverberating neural circuitry underlying their work memory disturbance. However, the extent to which verbal learning deficits in patients can be uniquely and specifically attributable to impaired synaptic plasticity remains to be elucidated. For example, in a randomized controlled trial (RCT) study of the differential effects of DCS on cognition in schizophrenia, Forsyth et al38 found a pattern of performance on tasks of working memory versus incremental or associative learning opposite to that observed in their prior studies of healthy participants.16 That is, their data indicated a DCS advantage in working memory but not in incremental learning in their patient sample.
While the precise contributions of NMDAR signaling to specific elements of the disease-related cognitive disturbance await further investigation, the current findings, nonetheless, do offer strong behavioral evidence for the centrality of associative learning deficit in schizophrenia, which was empirically distinct from, and not statistically confounded by, their impairment in working memory or by their general diminution in neuropsychological functioning. These findings point to the role verbal learning and memory impairment may play in the broad generalized deficit in chronic schizophrenia and may also help to understand the cognitive changes and vulnerabilities during the early premorbid phases of the illness, considering recent meta-analytic findings pointing to medium-to-large deficits on similar verbal tests of learning in individuals at clinical high risk for psychosis.13
Our findings also offered strong and robust evidence of the enduring and deleterious effects of overall negative symptoms on neuropsychological functioning across summary measures of intelligence and memory in this sample of patients with chronic schizophrenia. Indeed, in line with Bleuler’s formulation, the current study examined the role of a specific set of 3 symptoms—flat affect, anhedonia, and formal thought disturbance—in associative learning and memory performance for the patient group. Here, the results indicated that each of these symptoms correlated significantly with reduced VPA performance. However, hierarchical regression analyses revealed only flat affect contributed significantly to associative memory disturbance in schizophrenia, uniquely accounting for approximately 12.60%–14.98% of the variance in immediate recall scores. In contrast, more dramatic positive symptoms, namely hallucinations and delusions, which Bleuler characterized as accessory symptoms, did not correlate with any of the neuropsychological measures, including the VPA subtest of the WMS-III. Bleuler viewed the primary symptoms of schizophrenia as represented by this configuration of cognitive–affective–motivational disturbances, which he presumed arose from an underlying neuropathology distinct from that of delusions and hallucinations. The very limited brain imaging data of the current study did not allow for an examination of the underlying neural sources contributing to the breakdown of associative structure of thinking and emotion in chronic schizophrenia observed here. However, the archival data sample did include a relatively small subset of patients and controls (n = 39) who had both neuropsychological and MRI PFC gray matter volume measures. Although clearly statistically underpowered, PFC-VPA did correlate in the whole subset, with greater prefrontal gray matter volume linked to better associative learning and memory. This relationship comported with a well-established body of research implicating prefrontal circuitry in associative learning and memory as well as with network models that ascribe the PFC as a critical hub in large-scale network models of cortical function (eg, Bertolero et al39 and Warren et al40).
These findings are, however, qualified by several limitations of the current research design. First, the study, while employing rigorous multimodal assessment procedures, used an archival research design with the sample MRI and neuropsychological data collected over a time span of 24–30 months approximately 10 years ago. And although the behavioral data provided rather robust evidence of neuropsychological and symptom relationships consistent with Bleuler’s unitary theory of schizophrenia, the supporting MRI results were derived from a smaller subset of only those participants who had these measures available.19 In addition, these archival brain imaging studies were limited to the PFC and its subregions, and thus the contributions of other brain regions were not investigated. In addition, it is also important to point out that the associative impairment in this sample of patients occurred against a backdrop of clear evidence of their generalized cognitive deficit, underscoring that there are likely multiple informational and neural pathways underlying neuropsychological disturbance in schizophrenia. Nevertheless, taken together, these findings suggested that disturbance in associative thinking in chronic schizophrenia may be represented, neuropsychologically, by a fundamental deficit in PFC-supported verbal learning, and expressed, phenotypically, in heightened negative symptoms of flat affect and loss of motivation, initiation, and goal-directed behavior.
Acknowledgments
The authors acknowledge Professor Dean Salisbury for his thoughtful comments and suggestions. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the National Institute of Mental Health R21MH121704 (J.J.L.) and VA Merit Award CX000176 (M.E.S.).
References
- 1.Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International Universities Press; 1950. [Google Scholar]
- 2.McNally K. Myth and Forgetting: Bleuler’s “Four As”. In: A Critical History of Schizophrenia. Palgrave Studies in the Theory and History of Psychology. London: Palgrave Macmillan; 2016. doi: 10.1057/9781137456816_7 [DOI] [Google Scholar]
- 3.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–787. [DOI] [PubMed] [Google Scholar]
- 4.Kahn RS. On the origins of schizophrenia. Am J Psychiatry. 2020;177(4):291–297. [DOI] [PubMed] [Google Scholar]
- 5.Spence JT, Lair CV. Associative interference in the paired-associate learning of remitted and nonremitted schizophrenics. J Abnorm Psychol. 1965;70:119–122. [DOI] [PubMed] [Google Scholar]
- 6.Rushe TM, Woodruff PW, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophr Res. 1999;35(1):85–96. [DOI] [PubMed] [Google Scholar]
- 7.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in schizophrenia? A meta-analysis. Brain Cogn. 2003;53(2):121–124. [DOI] [PubMed] [Google Scholar]
- 8.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88(2):982–990. [DOI] [PubMed] [Google Scholar]
- 9.Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragland JD, Ranganath C, Barch DM, et al. . Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012;38(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher BA, Manschreck TC, Woods BT, Yurgelun-Todd DA, Tsuang MT. Frontal brain volume and context effects in short-term recall in schizophrenia. Biol Psychiatry. 1995;37(3):144–150. [DOI] [PubMed] [Google Scholar]
- 12.Oertel V, Kraft D, Alves G, et al. . Associative memory impairments are associated with functional alterations within the memory network in schizophrenia patients and their unaffected first-degree relatives: an fMRI study. Front Psychiatry. 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalan A, Salazar de Pablo G, Aymerich C, et al. . Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. [published online ahead of print June 16, 2021]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2021.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth JK, Lewis DA. Mapping the consequences of impaired synaptic plasticity in schizophrenia through development: an integrative model for diverse clinical features. Trends Cogn Sci. 2017;21(10):760–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdenis van Berlekom A, Muflihah CH, Snijders GJLJ, et al. . Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46(2):374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci USA. 2015;112(50):15331–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D.WMS-III: Wechsler Memory Scale Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 18.Nestor PG, Nakamura M, Niznikiewicz M, et al. . Attentional control and intelligence: MRI orbital frontal gray matter and neuropsychological correlates. Behav Neurol. 2015;2015:354186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtani T, Levitt JJ, Nestor PG, et al. . Prefrontal cortex volume deficit in schizophrenia: a new look using 3T MRI with manual parcellation. Schizophr Res. 2014;152(1):184–190. [DOI] [PubMed] [Google Scholar]
- 20.Allen TA, Fortin NJ. The evolution of episodic memory. Proc Natl Acad Sci USA. 2013;110(suppl. 2):10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20(7):1604–1612. [DOI] [PubMed] [Google Scholar]
- 22.Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388(6642):582–585. [DOI] [PubMed] [Google Scholar]
- 23.Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci USA. 2008;105(37):14228–14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 25.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D.Manual: Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 27.Andreasen N.Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- 28.Andreasen NC.Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 29.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31(2–3):113–120. [DOI] [PubMed] [Google Scholar]
- 31.Gold JM, Dickinson D.“Generalized cognitive deficit” in schizophrenia: overused or underappreciated? Schiz Bull. 2013;39(2):263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull. 2009;35(2):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestor PG, Kubicki M, Gurrera RJ, et al. . Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18(4):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22(2):246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Ye E, Asarnow RF. Effects of augmenting N-Methyl-D-aspartate receptor signaling on working memory and experience-dependent plasticity in schizophrenia: an exploratory study using acute d-cycloserine. Schizophr Bull. 2017;43(5):1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertolero MA, Yeo BT, D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci USA. 2015;112(49):E6798–E6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren DE, Power JD, Bruss J, et al. . Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci USA. 2014;111(39):14247–14252. [DOI] [PMC free article] [PubMed] [Google Scholar]