Figure 9 .
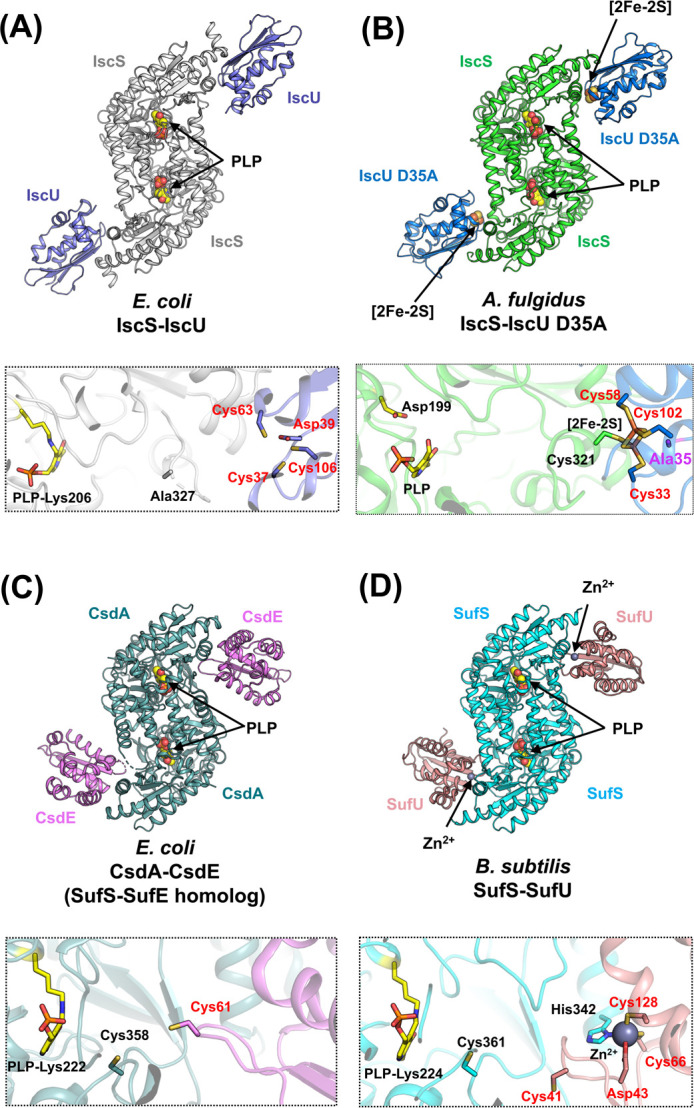
Overall structures and the close-up views of the interfaces of cysteine desulfurase-partner protein complexes. (A) E. coli IscS-IscU [PDB ID: 3lvl]. (B) A. fulgidus IscS-IscU D35A [PDB ID: 4eb5]. (C) E. coli CsdA-CsdE [PDB ID: 4lw4] (a SufS-SufE homolog). (D) B. subtilis SufS-SufU [PDB ID: 5xt5]. In the close-up views, key amino acid residues (except for Ala327 in IscS-IscU) and PLPs are shown as sticks. The loop region including Cys328 (the nucleophilic Cys in catalysis) in E. coli IscS is disordered. The black- and red-colored labels in the close-up views of the complex interfaces indicate the residues from the cysteine desulfurases and their partner proteins, respectively. The residue of Ala35 of A. fulgidus IscU D35A was shown in a stick model in magenta. It should be noted that the structure of A. fulgidus IscS-IscU D35A was modeled as a PLP-bound form, whereas the structure of A. fulgidus IscS alone harbored pyridoxamine-5'-phosphate (PMP) rather than PLP. The reason for the cofactors being different between A. fulgidus IscS-IscU D35A and A. fulgidus IscS is still elusive, but will be elaborated on in the section titled “An inactive cysteine desulfurase paralog with PMP rather than PLP”.
