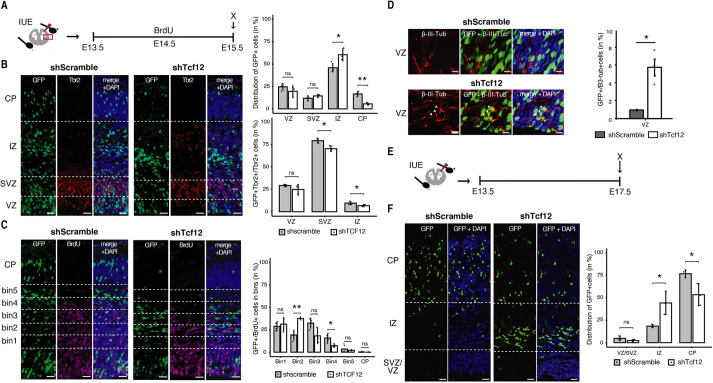
Fig. 5.
Loss of Tcf12 impairs neuronal migration during cortical development. (A) Experimental layout for the in utero electroporation (IUE) assay where IUE was carried out at E13.5 and analysed at E15.5. (B) Immunofluorescence images showing phenotypic changes following depletion of Tcf12. Here, E15.5 cortex electroporated at E13.5 with a control shscramble or a shTcf12 plasmid containing a GFP reporter (left panel) was analysed for the distribution of GFP+ and GFP+ Tbr2+/Tbr2+ cells (in %) across the cortical layers (right panel). (C) Neuronal migration was assessed by identifying five equidistant bins across the SVZ/IZ (left panel) and quantifying the distribution of GFP+/BrDU+ population (in %) in these bins (right panel). (D) Immunofluorescence images showing the expression of the neuron-specific marker β-III-Tubulin upon Tcf12 knockdown (left panel) and the distribution of GFP+/β-III-Tub+ cells (in %) in the ventricular zone (VZ) (right panel). (E) Experimental schematic for the in utero electroporation (IUE) assay where IUE was carried out at E13.5 and analysed at E17.5. (F) Immunofluorescence images showing phenotypic changes in the developing cortex following a prolonged knockdown of Tcf12 (left panel). The distribution of electroporated cortical cells (GFP+ cells) across the cortical layers is shown (in %) (right panel). Scale bars: 100 µm. Statistical significance was calculated using an unpaired two-tailed Student's t-test. *P<0.05, **P<0.01; n=3 or 4 independent biological replicates. Data are mean±s.d.