Summary
Background
A single dose strategy may be adequate to confer population level immunity and protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, especially in low- and middle-income countries where vaccine supply remains limited. We compared the effectiveness of a single dose strategy of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines against SARS-CoV-2 infection across all age groups and over an extended follow-up period.
Methods
Individuals vaccinated in North-West London, UK, with either the first dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines between January 12, 2021 and March 09, 2021, were matched to each other by demographic and clinical characteristics. Each vaccinated individual was additionally matched to an unvaccinated control. Study outcomes included SARS-CoV-2 infection of any severity, COVID-19 hospitalisation, COVID-19 death, and all-cause mortality.
Findings
Amongst matched individuals, 63,608 were in each of the vaccine groups and 127,216 were unvaccinated. Between 14 and 84 days of follow-up after matching, there were 534 SARS-CoV-2 infections, 65 COVID-19 hospitalisations, and 190 deaths, of which 29 were categorized as due to COVID-19. The incidence rate ratio (IRR) for SARS-CoV-2 infection was 0.85 (95% confidence interval [CI], 0.69 to 1.05) for Oxford-Astra-Zeneca, and 0.69 (0.55 to 0.86) for Pfizer-BioNTech. The IRR for both vaccines was the same at 0.25 (0.09 to 0.55) and 0.14 (0.02 to 0.58) for reducing COVID-19 hospitalization and COVID-19 mortality, respectively. The IRR for all-cause mortality was 0.25 (0.15 to 0.39) and 0.18 (0.10 to 0.30) for the Oxford-Astra-Zeneca and Pfizer-BioNTech vaccines, respectively. Age was an effect modifier of the association between vaccination and SARS-CoV-2 infection of any severity; lower hazard ratios for increasing age.
Interpretation
A single dose strategy, for both vaccines, was effective at reducing COVID-19 mortality and hospitalization rates. The magnitude of vaccine effectiveness was comparatively lower for SARS-CoV-2 infection, although this was variable across the age range, with higher effectiveness seen with older adults. Our results have important implications for health system planning -especially in low resource settings where vaccine supply remains constrained.
Keywords: Age, COVID-19, Hospitalisation, Mortality, SARS-CoV-2, Vaccination
Research in context.
Evidence before this study
Electronic searches of MEDLINE, EMBASE, medRxiv and the Cochrane Central Register of Controlled Trials were done for articles in English published between Jan 1, 2020 and September 21, 2021. Studies with the following characteristics were identified: (1) randomised trials or observational studies; (2) individuals with documented SARS-CoV-2 infection; (3) outcomes related to the effectiveness of the Oxford-AstraZeneca or Pfizer-BioNTech vaccine. While randomised control trials have tested a 2-dose strategy against SARS-CoV-2 infection, the efficacy of a single dose strategy remains less clear. Emerging observational studies that have shown efficacy of a single dose strategy against SARS-CoV-2 have been limited to short follow-up times and mainly older individuals.
Added value of this study
To our knowledge, this is the first study to use real-world observational data to compare the effectiveness of a single dose of the Oxford-AstraZeneca and Pfizer-BioNTech vaccines against COVID-19 over 3 months follow-up across the entire age spectrum in the general population. Single dose vaccination markedly reduced risk of hospitalisation or mortality from COVID-19. The magnitude of efficacy was less marked against SARS-CoV-2 following vaccination. However, we did observe substantial effect modification of vaccine effectiveness by age against SARS-CoV-2 infection of any severity; effectiveness was higher with increasing age.
Implications of all the available evidence
A single dose strategy, for both vaccines, was effective at reducing COVID-19 mortality and hospitalization rates. While a single dose strategy was associated with a relatively low reduction in the rate of SARS-CoV-2 infection at 3 months, there was significant effect modification by age, with higher vaccine effectiveness seen with older adults. Our results have important implications for health system planning, especially in low resource settings where vaccine supply remains constrained.
Alt-text: Unlabelled box
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has killed over 4.6 million worldwide as of September 01, 2021.1 Over 200 candidate vaccines against the virus which causes coronavirus disease 2019 (COVID-19) have been developed.1 The two most common COVID-19 vaccines used in vaccination campaigns worldwide are the Oxford-AstraZeneca (ChAdOx1 nCoV-19/AZD1222) and Pfizer-BioNTech (BNT162b2) COVID-19 vaccines. However, whilst randomised control trials have tested a 2-dose strategy against SARS-CoV-2 infection2, 3 the efficacy of a single dose strategy remains less clear. Emerging observational studies do suggest that a single dose vaccine strategy might confer high efficacy against SARS-CoV-2 infection and disease severity4, 5, 6, 7, 8: but these studies have been limited to a restricted age group – mainly older patients – with generally shorter follow-up of 4 weeks. To confer population level immunity and protection, especially in low- and middle-income countries where vaccine supply remains limited, a single dose strategy may be preferable. Fractional dosing, where individuals receive less than the recommended dosage of vaccine, has been successfully utilised in previous outbreaks of other infectious diseases, including cholera9 and yellow fever.10 If shown to be effective, a single dose COVID-19 campaign in countries where there are less supplies would be easier to implement logistically, with the potential to reach herd immunity more rapidly,11, 12, 13
A single dose strategy may also be preferable where large populations need to be vaccinated in a shorter time period. As such, the United Kingdom following advice from the Joint Committee on Vaccination and Immunisation recommended prioritizing a first vaccine dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines for all eligible individuals prior to securing a second vaccine dose for recipients.14
Using high fidelity routine health care data with linkage to outcomes, we evaluated and compared the effectiveness of a single dose vaccine strategy of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines against SARS-CoV-2 infection across all age groups and over longer follow up periods.
Methods
Study population
We analysed anonymised data accessed in the Imperial Clinical Analytics, Research and Evaluation (iCARE) environment,15 which holds a unique integrated care data set including the pre-established Whole System Integrated Care (WSIC) data collated for the purposes of population health in the sector, capturing all adults who are registered with a general practitioner, or who have a resident postcode, in the North-West London (NWL) catchment area.16 This study was undertaken within a research database, using data fully anonymized data, which was approved by the West Midlands - Solihull Research Ethics Committee. REC reference: 18/WM/0323.
Data sources
The WSIC database includes demographic and clinical data from general practice (GP) clinical systems (SystmOne, eMIS), SARS-CoV-2 PCR pathology results from NWL Pathology, The Doctors Laboratory (TDL) and national SARS-CoV-2 test results, and hospital admission data from secondary uses service (SUS) data feeds. Data on vaccination and SARS-CoV-2 testing was available within hours of the data being recorded in source data systems. The GP clinical systems, which have been almost fully computerised since the early 1990s, provide a longitudinal electronic healthcare record of all primary care encounters including information on Quality and Outcome Framework (QOF) indicators. The clinical domain of the QOF, which identifies indicators across 19 clinical areas, including chronic kidney disease, hypertension, and heart failure, was used to identify chronic conditions for each individual. All data were de-identified prior to access for research.
Study design
We designed this observational cohort study to assess the effect of a single dose of the Oxford-AstraZeneca and Pfizer-BioNTech vaccines on outcomes related to vaccine effectiveness. Eligibility criteria included individuals aged 16 years or older who were alive on December 08, 2020, at the start of the UK COVID-19 vaccination campaign. Participants were excluded if they previously had a documented positive SARS-CoV-2 polymerase-chain-reaction (PCR) test, were administered the Modena vaccine or had missing data.
Data were available for 20 weeks following the onset of the vaccination program. However, due to vaccine supply issues and safety concerns about vaccines at different time points, both the first dose of both vaccines were administered in large numbers only during weeks 5–12. As our aim was to match individuals between vaccine groups and those unvaccinated on a week-by-week basis, we only matched individuals during weeks 5–12 (January 12, 2021–March 09, 2021).
The matching process is covered in detail in the Supplementary Material. In summary, the eligible vaccinated and unvaccinated persons were matched on a week-by-week basis on the following variables: age (in bins of two consecutive years), gender (male and female), ethnic category (white, south Asian, black, other Asian, other, not recorded/stated), the number of SARS-CoV-2 tests from December 8th 2020 until the start of the relevant week of matching, care home residence, Index of Multiple Deprivation decile, number of comorbidities, clinically extremely vulnerable status, and being a 16–64 year old with underlying health conditions.
From this cohort of matched individuals, we then matched an individual that received the Oxford-AstraZeneca vaccine with an individual receiving the Pfizer-BioNTech vaccine on a week-by-week basis using the same matching variables as above. Following this week-by-week matching process, each matched group would contain two individuals that had not been vaccinated by the start of that week, as well as one individual that had received the Oxford-AstraZeneca vaccine and one individual that received the Pfizer-BioNTech vaccine during the same week.
For each person, follow-up ended at the earliest of the following events: occurrence of an outcome event, death, vaccination (for unvaccinated controls), vaccination of the matched control (for vaccinated persons), or vaccination with the second dose (for vaccinated persons) or the end of the study period.
Outcomes
The four outcomes of interest were documented SARS-CoV-2 infection of any severity confirmed by a positive PCR test, COVID-19 hospitalisation, COVID-19 death, and all-cause mortality. Hospitalisation related to COVID-19 was defined as patients admitted to hospital who had tested positive for SARS-CoV-2 in the last 14 days or patients with a positive COVID-19 test result during an inpatient stay. Death from COVID-19 was defined as deaths among individuals with a positive SARS-CoV-2 test that occurred within 28 days of the test (according to Public Health England guidance17).
Statistical analysis
For each group (unvaccinated, Oxford-AstraZeneca or Pfizer-BioNTech), we calculated the number and rate of events per 1000 person-years for each outcome of interest.
The period immediately after the first dose when immunity is gradually building,2 was excluded in the main analyses using a 14-day landmark. We also calculated incidence rate ratios (IRRs) comparing the rates of (1) those receiving Oxford-AstraZeneca versus those unvaccinated, (2) those receiving Pfizer-BioNTech versus those unvaccinated, and (3) those receiving Pfizer-BioNTech versus those receiving Oxford-AstraZeneca. For the analyses of days 14–84 of follow-up, persons were excluded if they or a person they were matched with were censored before the start of the follow-up period (day 14) to maintain balance between the groups (each matching group contains one person receiving Oxford-AstraZeneca, one receiving Pfizer-BioNTech, and two unvaccinated).
Survival curves for the vaccinated and unvaccinated groups were estimated with the Kaplan–Meier estimator.18 Restricted cubic splines were used to display the association between age and COVID-19 outcomes at 84 days after first dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines. Analyses were performed using Stata (version 16.1) and R (version 3.5.0). The study is reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Guidelines.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing the report. AK, AT and AS had access to all data and take full responsibility for its integrity and the accuracy of the analyses. The corresponding author had final responsibility for the decision to submit for publication.
Results
Study population
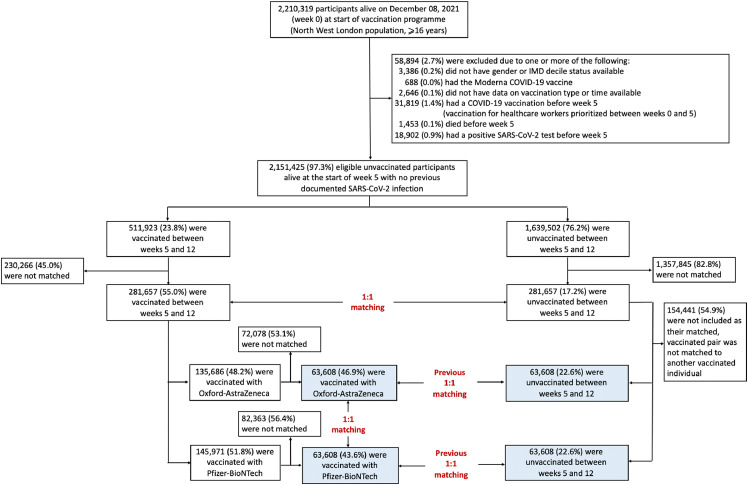
Of 2210319 participants who were alive on December 08, 2020, 2151425 were eligible for the study and 281,657 vaccinated individuals were matched to unvaccinated controls (Figure 1). From this cohort of matched individuals, 63,608 were matched in each vaccine group. The final dataset therefore had 127,216 vaccinated participants (63,608 Oxford-AstraZeneca and 63,608 Pfizer-BioNTech) and 127,216 unvaccinated individuals that had previously been matched to these vaccinated persons (Figure 1). The demographic and clinical characteristics for all eligible and matched individuals, stratified by whether or not they were vaccinated, are summarised in Table S1.
Figure 1.
Study population and cohort enrolment process.
Matched individuals were younger (median age 53 years (interquartile range [IQR], 41 to 61)) than the eligible population who were vaccinated (61, 48 to 71) and had a lower prevalence of chronic conditions because there was a smaller pool of potential unvaccinated matches for older vaccine recipients, owing to high vaccination rates in the older population. The baseline characteristics of matched individuals are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the matched populations at baseline that were unvaccinated or received a single dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines.
| Unvaccinated (N = 127,216) | Oxford-AstraZeneca (N = 63,608) | Pfizer (N = 63,608) | |
|---|---|---|---|
| Age in years [median (IQR)] | 53 (41–61) | 53 (41–61) | 53 (41–61) |
| Male [n(%)] | 58,796 (46.2%) | 29,398 (46.2%) | 29,398 (46.2%) |
| At risk age 16–64 [n(%)] | 12,938 (10.2%) | 6469 (10.2%) | 6469 (10.2%) |
| Care home resident [n(%)] | 6 (0.005%) | 3 (0.005%) | 3 (0.005%) |
| Clinically extremely vulnerable [n(%)] | 2114 (1.7%) | 1057 (1.7%) | 1057 (1.7%) |
| Ethnicity [n(%)] | |||
| White | 58,644 (46.1%) | 29,322 (46.1%) | 29,322 (46.1%) |
| South Asian | 28,252 (22.2%) | 14,126 (22.2%) | 14,126 (22.2%) |
| Black | 8464 (6.7%) | 4232 (6.7%) | 4232 (6.7%) |
| Other Asian | 11,692 (9.2%) | 5846 (9.2%) | 5846 (9.2%) |
| Mixed/other | 8278 (6.5%) | 4139 (6.5%) | 4139 (6.5%) |
| Not reported/recorded | 11,886 (9.3%) | 5943 (9.3%) | 5943 (9.3%) |
| Comorbidities [n(%)] | |||
| 0 | 74,912 (58.9%) | 37,456 (58.9%) | 37,456 (58.9%) |
| 1 | 30,228 (23.8%) | 15,114 (23.8%) | 15,114 (23.8%) |
| 2 | 14,680 (11.5%) | 7340 (11.5%) | 7340 (11.5%) |
| 3 | 5076 (4.0%) | 2538 (4.0%) | 2538 (4.0%) |
| 4+ | 2320 (1.8%) | 1160 (1.8%) | 1160 (1.8%) |
| IMD decile [n(%)] | |||
| 1 | 1372 (1.1%) | 686 (1.1%) | 686 (1.1%) |
| 2 | 12,308 (9.7%) | 6154 (9.7%) | 6154 (9.7%) |
| 3 | 19,244 (15.1%) | 9622 (15.1%) | 9622 (15.1%) |
| 4 | 22,548 (17.7%) | 11,274 (17.7%) | 11,274 (17.7%) |
| 5 | 20,804 (16.4%) | 10,402 (16.4%) | 10,402 (16.4%) |
| 6 | 17,396 (13.7%) | 8698 (13.7%) | 8698 (13.7%) |
| 7 | 11,828 (9.3%) | 5914 (9.3%) | 5914 (9.3%) |
| 8 | 11,896 (9.4%) | 5948 (9.4%) | 5948 (9.4%) |
| 9 | 7206 (5.7%) | 3603 (5.7%) | 3603 (5.7%) |
| 10 | 2614 (2.1%) | 1307 (2.1%) | 1307 (2.1%) |
| Number of tests before week of matching [n(%)] | |||
| 0 | 124,566 (97.9%) | 62,283 (97.9%) | 62,283 (97.9%) |
| 1 | 2394 (1.9%) | 1197 (1.9%) | 1197 (1.9%) |
| 2+ | 256 (0.2%) | 128 (0.2%) | 128 (0.2%) |
| Comorbidities [n(%)] | |||
| Anxiety | 13,631 (10.7%) | 4842 (7.6%) | 4505 (7.1%) |
| Asthma | 122,626 (3.6%) | 3947 (6.2%) | 4245 (6.7%) |
| Atrial fibrillation | 796 (0.6%) | 459 (0.7%) | 584 (0.9%) |
| Cancer | 1821 (1.4%) | 1184 (1.9%) | 1336 (2.1%) |
| CKD | 782 (0.6%) | 419 (0.7%) | 517 (0.8%) |
| COPD | 913 (0.7%) | 507 (0.8%) | 564 (0.9%) |
| Dementia | 195 (0.2%) | 124 (0.2%) | 87 (0.1%) |
| Depression | 13,441 (10.6%) | 4882 (7.7%) | 4561 (7.2%) |
| Diabetes | 8153 (6.4%) | 6355 (10.0%) | 6807 (10.7%) |
| Heart failure | 340 (0.3%) | 187 (0.3%) | 198 (0.3%) |
| Hypertension | 19,014 (15.0%) | 9258 (14.6%) | 9046 (14.2%) |
| Hypothyroidism | 6505 (5.1%) | 2748 (4.3%) | 2557 (4.0%) |
| IHD | 2085 (1.6%) | 1321 (2.1%) | 1337 (2.1%) |
| Learning disability | 241 (0.2%) | 225 (0.4%) | 162 (0.3%) |
| Mental health | 2282 (1.8%) | 642 (1.0%) | 559 (0.9%) |
| Obesity | 8554 (6.7%) | 4559 (7.2%) | 4588 (7.2%) |
| PAD | 273 (0.2%) | 182 (0.3%) | 134 (0.2%) |
| Palliative care | 144 (0.1%) | 86 (0.1%) | 50 (0.1%) |
| Parkinson's | 102 (0.1%) | 54 (0.1%) | 54 (0.1%) |
| Rheumatoid arthritis | 568 (0.4%) | 312 (0.5%) | 354 (0.6%) |
| Stroke or TIA | 1056 (0.8%) | 551 (0.9%) | 533 (0.8%) |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease; IHD, Ischaemic heart disease; IQR, Interquartile range; TIA, Transient ischaemic attack.
The median follow-up time for the matched unvaccinated group was 70 days (IQR, 42 to 84), Oxford-AstraZeneca 70 days (63 to 77), and Pfizer-BioNTech 70 days (63 to 77). Table S2 shows the time interval between the first and second dose for those vaccinated, with 69.0% in the Oxford-AstraZeneca group and 63.3% in the Pfizer-BioNTech group not having received a second dose by their censoring date.
During the period from 14 to 84 days, individuals in the matched unvaccinated group had a SARS-CoV-2 test documented at a rate of 163.95 (95% CI, 157.92 to 170.20) per 1000 person-years to the first testing event, whilst the rates were higher at 284.34 (273.49 to 295.63) in the Oxford-AstraZeneca group and 250 (240.25 to 261.18) in the Pfizer-BioNTech group. A similar trend with higher testing rates in both vaccine groups compared to the unvaccinated group was seen across all ages (Table S3).
Vaccine effectiveness
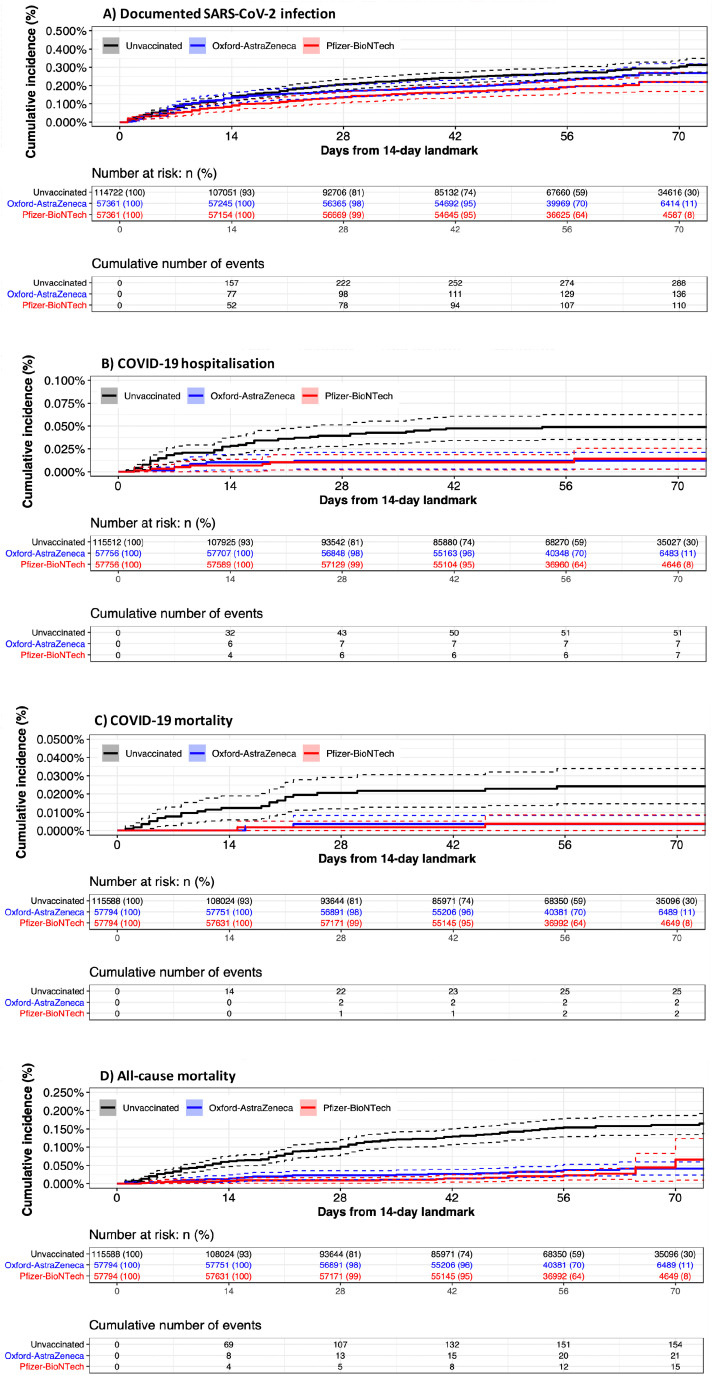
During the period from 14 to 84 days, 534 infections were documented overall, of which 65 (11.9%) required hospitalization, and 29 (5.6%) resulted in death. There were 190 deaths from any cause during this period Figure 2. shows the cumulative incidence curves for the outcomes during the time period from 14 to 84 days for the matched groups Table 2. shows the corresponding number of events and estimated vaccine effectiveness. All outcomes were less common among vaccinated individuals than those unvaccinated. The rate of documented SARS-CoV-2 infection per 1000 person-years was 15.1 and 12.4 for the Oxford-AstraZeneca and Pfizer-BioNTech vaccines, respectively, compared to 18.2 for unvaccinated individuals. For COVID-19 hospitalization, the rates were overall lower at 3.2, 0.8 and 0.8 for the unvaccinated, Oxford-AstraZeneca and Pfizer-BioNTech vaccine groups, respectively.
Figure 2.
Cumulative incidence of COVID-19 outcomes at 14 to 84 days after first dose of Oxford-AstraZeneca and Pfizer-BioNTech vaccines.
Table 2.
Estimated vaccine effectiveness against COVID-19 outcomes 14 to 84 days after matching of the first dose of Oxford-AstraZeneca and Pfizer-BioNTech vaccines.
| Documented SARS-CoV-2 infection | COVID-19 hospitalisation* | COVID-19 mortality ¥ | All-cause mortality | |
|---|---|---|---|---|
| Number of events (rate per 1000 person-years [95%CI]) | ||||
| Unvaccinated [U] | 288 (18.23 [16.24 to 20.46]) |
51 (3.20 [2.43 to 4.21]) |
25 (1.57 [1.06 to 2.32]) |
154 (9.65 [8.24 to 11.31]) |
| Oxford-AstraZeneca [AZ] | 136 (15.11 [12.77 to 17.88]) |
7 (0.77 [0.37 to 1.62]) |
2 (0.22 [0.06 to 0.88]) |
21 (2.31 [1.51 to 3.55]) |
| Pfizer-BioNTech [P] | 110 (12.44 [10.32 to 14.99]) |
7 (0.78 [0.37 to 1.65]) |
2 (0.22 [0.06 to 0.90]) |
15 (1.68 [1.01 to 2.79]) |
| Incidence rate ratios (95%CI) – p-value) | ||||
| AZ vs U | 0.85 (0.69 to 1.05) - p = 0.121 | 0.25 (0.09 to 0.55) - p < 0.001 | 0.14 (0.02 to 0.58) - p < 0.001 | 0.25 (0.15 to 0.39) - p < 0.001 |
| P vs U | 0.69 (0.55 to 0.86) - p < 0.001 | 0.25 (0.09 to 0.55) - p < 0.001 | 0.14 (0.02 to 0.58) - p < 0.001 | 0.18 (0.10 to 0.30) - p < 0.001 |
| P vs AZ | 0.81 (0.62 to 1.05) - p = 0.098 | 1.00 (0.30 to 3.34) - p = 1.000 | 1.00 (0.07 to 13.80) - p = 1.000 | 0.71 (0.34 to 1.45) - p = 0.324 |
COVID-19 hospitalisation defined as having a positive documented SARS-CoV-2 test 14 days before or within 7 days of being admitted to hospital.
COVID-19 mortality defined as having a positive documented SARS-CoV-2 test 28 days prior to death.
The IRRs for each outcome comparing the Oxford-AstraZeneca, Pfizer-BioNTech and unvaccinated groups are shown in Table 2 for days 14–84 of follow-up. The IRR for documented SARS-CoV-2 infection was 0.85 (95% CI, 0.69 to 1.05) for the Oxford-Astra-Zeneca vaccine, and 0.69 (0.55 to 0.86) for the Pfizer-BioNTech vaccine. The IRR for both vaccines was the same at 0.25 (0.09 to 0.55) and 0.14 (0.02 to 0.58) for reducing COVID-19 hospitalization and COVID-19 mortality, respectively. The IRR for all-cause mortality was 0.25 (0.15 to 0.39) for the Oxford-Astra-Zeneca vaccine, and 0.18 (0.10 to 0.30) for the Pfizer-BioNTech vaccine.
The IRRs comparing the Oxford-AstraZeneca and Pfizer-BioNTech vaccine groups are shown in Table 2 for days 14–84 of follow-up. There were no significant differences between the two vaccinated groups for any of the outcomes.
Vaccine effectiveness in different age groups
Figure 3 shows the association between age and COVID-19 outcomes after the first dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines over the 14 to 84 day follow-up period.
Figure 3.
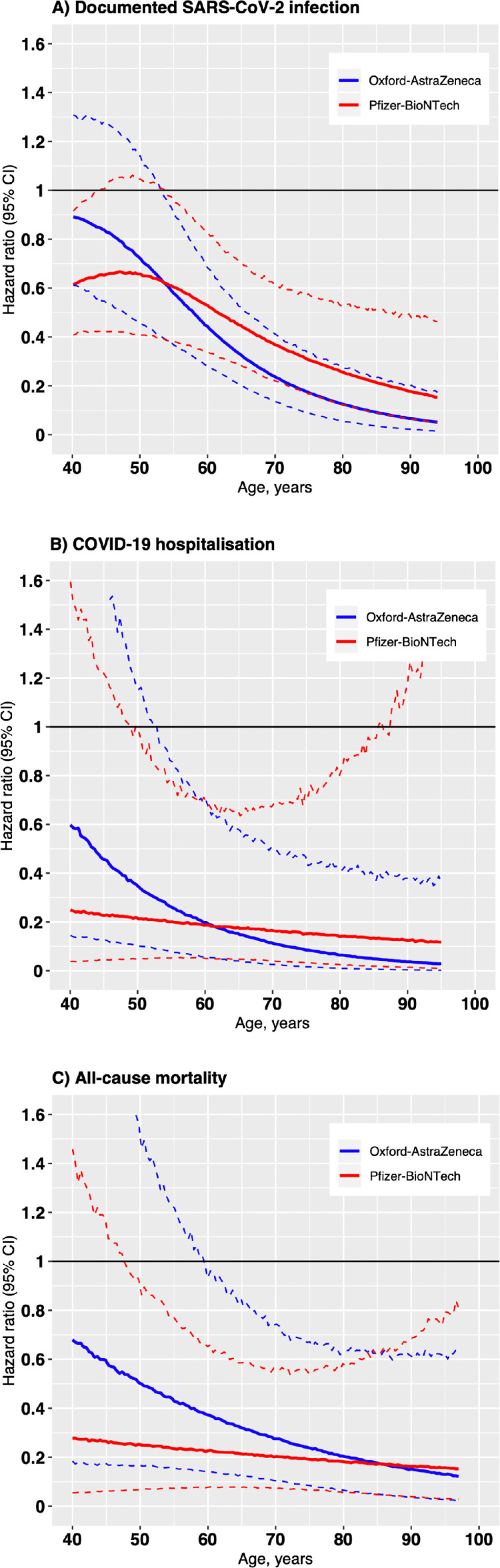
Association between age and COVID-19 outcomes after the first dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines over the 14 to 84 day follow-up period
Restricted cubic splines displaying hazard ratios of COVID-19 outcomes with 95% confidence intervals (dotted lines). The reference for each hazard ratio was the outcome in the unvaccinated group at corresponding ages. The 95% confidence intervals (dotted lines) are based on 10,000 predictions from the model using bootstrapped samples of the corresponding age-specific outcome counts. The spline for documented SARs-CoV-2 infection was adjusted by testing rate at baseline.
Age was an effect modifier of the association between vaccination and SARS-CoV-2 infection of any severity; lower hazard ratios for increasing age. In 40 year olds, the hazard ratio for SARS-CoV-2 infection was 0.89 (0.61 to 1.3) and 0.62 (0.41 to 0.91) for the Oxford-AstraZeneca and Pfizer-BioNTech vaccine, respectively, falling to 0.06 (0.02 to 0.19) and 0.18 (0.06 to 0.48) in those aged 90 years. Vaccine effect modification by age was however less apparent for outcomes related to severe COVID-19 infection (Figure 3B and C). We were unable to assess for effect modification by age on COVID-19 mortality due to low event rates. While there was a trend towards the Pfizer-BioNTech vaccine having a lower hazard ratio than the Oxford-AstraZeneca vaccine in younger individuals (Figure 3), the number of events among the young was low so confidence intervals were wide.
Discussion
To our knowledge, this is the first study to use real-world observational data to compare the effectiveness of a single dose of the Oxford-AstraZeneca and Pfizer-BioNTech vaccines against COVID-19 over 3 months follow-up across the entire age spectrum in the general population. While the Oxford-AstraZeneca and Pfizer-BioNTech vaccines as a single dose were associated with a relatively low reduction in the rate of SARS-CoV-2 infection at 3 months of 15% and 31% compared to being unvaccinated, respectively, the effect estimate crossed the null for the Oxford-AstraZeneca vaccine. Vaccination with either vaccine was however associated with a marked reduction in outcomes related to severe COVID-19 infection. We observed effect modification in vaccine effectiveness by age across both the Oxford-AstraZeneca and Pfizer-BioNTech vaccines. A single dose of either vaccine to prevent SARS-CoV-2 infection was more effective with older age. This effect modification was less apparent for outcomes related to severe COVID-19 infection. No difference in effectiveness after a single dose was observed between the vaccines at 3 months follow-up.
COVID-19 vaccination has begun in several countries, with many more countries starting in the upcoming months. The current COVID-19 vaccination strategies of most countries consider vaccination with full dosage (two doses), but some, including the UK, are vaccinating twice as many people with a single dose and delaying the second dose.14 While vaccine supply shortages are less of an issue in high-income countries, the situation is less certain in low- and middle-income countries, where vaccine supplies are arriving in smaller quantities at later times.19 Mathematical models exploring the use of single dose campaigns have shown that optimal use of resources depends primarily on the level of single dose efficacy.13,20
The efficacy of a single vaccine dose against ‘symptomatic’ COVID-19 infection has been reported in large randomised controlled trials (RCTs) as 52% (95%CI, 30 to 68%) from day 1 to 21 after a single dose of the Pfizer-BioNTech vaccine2 and 76% (95% CI, 59.3 to 95.9%) from day 22 to 90 after a single dose of the Oxford-AstraZeneca vaccine in exploratory analyses.21 These studies were not designed to assess the efficacy of a single dose regimen. Individuals were vaccinated with their second dose at 21 days in the Pfizer-BioNTech trial.2 We observed a single dose vaccine effectiveness of 15% (-5 to 31) for the Oxford-Astra-Zeneca vaccine, and 31% (14 to 45) for the Pfizer-BioNTech vaccine between 14 and 84 days. It is difficult to compare results from these RCTs and our study due to differences in population characteristics, follow-up duration and the specific outcomes measured. Moreover, the differences in vaccine effectiveness reported between the Phase III trials and our study may additionally be due to variation in exposure to different SARS-CoV-2 variants.
While overall, the effect estimate was lower for both vaccines compared to trial results, there was effect modification with increasing vaccine effectiveness of a single dose in older individuals. In 40-year-olds, the effectiveness for preventing SARS-CoV-2 infection was 11% (-30 to 39) for the Oxford-AstraZeneca vaccine and 38% (95% CI, 9 to 59) for the Pfizer-BioNTech vaccine. The effectiveness increased to 94% (81 to 98) and 82% (52 to 94), respectively, in those aged 90 years.
Vaccine effectiveness was overall higher for more serious outcomes reaching 75% (45 to 91) and 86% (42 to 98) for reducing COVID-19 hospitalization and COVID-19 mortality, respectively, for both vaccines at 84 days follow-up. There was little effect modification due to age for the Pfizer-BioNTech vaccine for severe outcomes, although the Oxford-AstraZeneca vaccine seemed to be less effective in younger individuals. Neither of the vaccine trials stratified analyses for vaccine effectiveness after the first dose by age group.2,21
Other real world analyses of the effectiveness of either the Oxford-AstraZeneca or Pfizer-BioNTech COVID-19 vaccines are now emerging, which similarly demonstrate single dose vaccine effectiveness despite differences in study design, population characteristics and follow-up duration between all studies.8,22,23 An analysis of data on over 1.1 million individuals showed that an effect of 60% for documented SARS-CoV-2 infection and 78% for COVID-19 hospital admission was found at 21–27 days after the first dose of the Pfizer-BioNTech vaccine.8 A test-negative case-control design has reported that single dose of either the Oxford-AstraZeneca or Pfizer-BioNTech vaccine had 60–70% protection against symptomatic COVID-19 and about 80% effectiveness against COVID-19 hospital admission.22 The analysis was restricted to those over the age of 70 with the median follow-up being less than 4 weeks for both vaccines. Similarly, a single dose of the Pfizer-BioNTech vaccine has been shown to be effective in reducing the risk of SARS-CoV-2 infection by two-thirds in adults ≥70 years old in British Columbia, Canada, at 5-weeks follow-up.24 At 84 days follow-up in our study, we found a comparable vaccine effectiveness against COVID-19 admission in those over the age of 70 (Figure 3B).
A recent prospective cohort study from Scotland found the first dose of the Pfizer-BioNTech vaccine was associated with a vaccine effect of 91% (95% CI, 85 to 94) against COVID-19 hospital admission at 28–34 days post-vaccination. Vaccine effect at the same time interval for the Oxford-AstraZeneca vaccine was 88% (95% CI, 75 to 94).23 Although limited to stratifying individuals in to three age groups rather than assessing the interaction of age as a continuous variable, they showed a similar trend to the present study of effect modification of vaccine effectiveness by age, with higher vaccine effectiveness in the oldest age group. Vaccine effect modification by age may be due to multiple reasons. While seroconversion rates and quantitative antibody levels after a single dose have been shown to be lower in older individuals, especially in those aged >60 years,25 differences in potential behavioural effects associated with vaccination, specifically behavioural disinhibition, with lower perceived risk, may lead to higher SARS-CoV-2 transmission rates in the young.26 These results therefore suggest that people should continue to wear face masks and maintain physical distance after the first vaccine to prevent onward transmission. In addition, our study period included the early part of the vaccination campaign when the proportion of young individuals receiving the first dose of the vaccine was low.27
Effect modification of vaccine effectiveness by age was most apparent against SARS-CoV-2 infection of any severity rather than for outcomes related to severe COVID-19 infection; COVID-19 hospitalisation, and all-cause mortality. This finding may be explained by a three-way interaction between age, COVID-19 severity and vaccine effectiveness. As older individuals are more likely to have severe COVID-19 infection,28 which was shown in our study to be less likely following single dose vaccination, vaccine effectiveness for all SARS-CoV-2 infections will appear to increase with age.
The main limitations of our study relate to its observational nature. As with any observational study, our results may have been affected by residual confounding due to differences between vaccinated and unvaccinated individuals, especially with regards to health-seeking behaviour and risk exposure. Despite precise matching on multiple demographic and clinical factors, including pre-matching test seeking behaviour, unmeasured confounders may still have influenced our results, including relevant social contact patterns. To account for variation in SARS-CoV-2 exposure rates during follow-up, individuals in the unvaccinated, Oxford-AstraZeneca and Pfizer-BioNTech vaccines were matched on a week-by-week basis to align local exposure rates between matched individuals during follow-up. While we found the SARS-CoV-2 testing rate was higher in the vaccinated groups than the unvaccinated group, which may lead to an underestimation of vaccine effectiveness for SARS-CoV-2 related outcomes, the testing rate was similar between the vaccine groups. Our rigorous week-by-week matching process came at the cost of not including in the final cohort approximately 50% of the vaccinated persons who otherwise met the study's eligibility criteria.
We were unable to assess single dose vaccine effectiveness beyond 84 days, when waning of vaccine effectiveness may become an issue. It is difficult to assess single dose vaccine effectiveness far beyond 84 days in the UK as the JCVI advised that the second dose of the Pfizer-BioNTech vaccine and Oxford-AstraZeneca vaccine should be given between 3 to 12 and 4 to 12 weeks following the first-dose, respectively. As highlighted in Table S2, approximately 25% of matched individuals in both the Pfizer-BioNTech and Oxford-AstraZeneca groups already had the second vaccination dose by 84 days. In Canada, first and second doses of vaccines against SARS-CoV-2 were uniquely spaced 16 weeks apart. Whilst a single dose of the Pfizer-BioNTech vaccine has been shown to be effective in reducing the risk of SARS-CoV-2 infection by at least three-quarters at 16 weeks follow-up, this was only assessed in the healthcare worker population, which are likely to have different health-seeking behaviour and exposure risk than the general population.29
The vaccine effectiveness estimates calculated are an average over multiple SARS-CoV-2 variants. Data on specific SARS-CoV-2 isolates during PCR testing was not available. While several SARS-CoV-2 variants of concern have been identified in the UK, during the study period, the B.1.1.7 variant was the most dominant variant. This is particularly relevant as the B.1.1.7 variant has been detected in over 40 countries worldwide,30 and the major Phase III vaccine trials were conducted before this strain was dominant.2,3 Data on whether vaccine-induced immunity can protect against multiple variants are limited. Based on preliminary reports from efficacy trials and immunogenicity studies, COVID-19 vaccines likely retain some efficacy against multiple variants.31,32 Since the end of May 2021, infections compatible with the Delta variant have been the most common in England and in recent weeks in all four UK countries. The picture emerging from various countries does, however, suggest that the vaccines may be less efficacious against the Delta variant for symptomatic infection compared with earlier forms of the virus.33,34 Despite these drops in efficacy, the Oxford-AstraZeneca and Pfizer-BioNTech vaccines have been shown to all reduce the risk of death by more than 85%, regardless of variant.34 While reports suggest a similar level of effectiveness for severe COVID-19 infection against the Delta variant as observed in our study, further research in the upcoming months is required to assess the effect of the Oxford-AstraZeneca and Pfizer-BioNTech vaccines on the Delta variant.
Neither of the vaccines are intended to be given as a single dose. Although we had a large population sample, there were insufficient number of individuals who had received the second dose of either the Oxford-AstraZeneca or Pfizer-BioNTech vaccines to reliably study vaccine effectiveness after receiving the full two-dose schedule. Assessing vaccine effectiveness of a single vaccine dose is of health policy interest worldwide given the uncertainty over whether a second dose of each vaccine should be deferred to allow more rapid coverage of the broader global population. While successful immunisation programmes generally result from high vaccine effectiveness with full dosage, fractional dosing with a single dose may be relevant in populations that are difficult to reach, or where background seroprevalence rates are high.
While the Oxford-AstraZeneca and Pfizer-BioNTech vaccine as a single dose was associated with a relatively low reduction in the rate of SARS-CoV-2 infection at 3 months, there was significant effect modification by age, with higher effectiveness seen with older adults. A single dose of either vaccine was more effective for preventing more serious outcomes across all ages. No difference in effectiveness after a single dose was observed between the vaccines at 3 months follow-up. Our results provide an important metric for health system planning especially in low- and middle-income countries.
Contributors
AK, AT, UB, BG, EM and JM conceived the hypothesis. AK and AT wrote the study protocol. BG, AM, LM, and AK carried out the programming to extract the data and curate the dataset. AK, AT and AM had access to and verified the dataset. AK, AT and AS undertook all data analyses. AK and AT drafted the manuscript and revised it in response to reviewer comments. AT, AS, BG, AM, LM, SG, SB, PA, TS, IG, JR, KS, EM and JM provided a critical review of the manuscript. All authors read and approved the final version of the manuscript. AK is the guarantor for this paper. The corresponding author, AK, attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Funding
National Institute for Health Research Imperial Biomedical Research Centre, British Heart Foundation and Wellcome Trust.
Data sharing statement
We are unable to extract or publish patient level data from the iCARE environment due to data protection restrictions. Any request to access data can be made to Nwlccgs.covid19IG@nhs.net referring to the title of this paper. The research has used WSIC data which is an integrated care data platform held in NWL region. WSIC de-identified data has Health Research Authority approval until 2023 which enables the use of this for research purposes for any retrospective studies approved by the NWL data access sub-group. This research was approved by the committee in June 2021. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people's patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone's privacy, and it's important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives.
Declaration of interests
This research was enabled by the iCARE environment and WSIC team and data resources (https://imperialbrc.nihr.ac.uk/facilities/icare/). The research was supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC), the NIHR Imperial Patient Safety Translational Research Centre and the NWL NIHR Applied Research Collaboration. AK is funded by a British Heart Foundation clinical research training fellowship (FS/20/18/34972). AT is funded by a Sir Henry Wellcome Postdoctoral Fellowship (222770/Z/21/Z). JM is supported by the BHF Imperial Centre for Research Excellence (RE/18/4/34215). All other authors have none to declare.
Acknowledgments
The authors would like to acknowledge members of the NWL Data & Analytics Gold Command for contribution to interpretation of the findings; Roger Chinn, Martin Kuper, Merav Dover, James Biggin-Lamming, Sanjay Gautama, Kevin Jarrold. The authors would also like to acknowledge Owain Griffiths from WSIC for helping with data access and data quality checks. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2022.101344.
Appendix. Supplementary materials
References
- 1.Johns Hopkins University and Medicine. Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html. Accessed January 1 2022
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and effectiveness of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and effectiveness of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AstraZeneca. AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19. https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html. Accessed January 1 2022
- 5.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;6736:18–19. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodick G., Tene L., Patalon T., et al. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Eng J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreras E., Chizema-Kawesha E., Blake A., et al. Single-dose cholera vaccine in response to an outbreak in zambia. N Engl J Med. 2018;378:577–579. doi: 10.1056/NEJMc1711583. [DOI] [PubMed] [Google Scholar]
- 10.Casey R.M., Harris J.B., Ahuka-Mundeke S., et al. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak - final report. N Engl J Med. 2019;381(5):444–454. doi: 10.1056/NEJMoa1710430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnabas R.V., Wald A. A public health covid-19 vaccination strategy to maximize the health gains for every single vaccine dose. Ann Int Med. 2021;174(4):552–553. doi: 10.7326/M20-8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paltiel A.D., Zheng A., Schwartz J.L. Speed versus efficacy: quantifying potential tradeoffs in COVID-19 vaccine deployment. Ann Int Med. 2021;174:568–570. doi: 10.7326/M20-7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuite A.R., Zhu L., Fisman D.N., Salomon J.A. Alternative dose allocation strategies to increase benefits from constrained covid-19 vaccine supply. Ann Int Med. 2021;174(4):570–572. doi: 10.7326/M20-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Kingdom Department of Health and Social Care. Joint committee on vaccination and immunisation: advice on priority groups for COVID-19 vaccination. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020. Accessed January 1 2022
- 15.Imperial Biomedical Research Centre. Imperial clinical analytics, research and evaluation (iCARE). https://imperialbrc.nihr.ac.uk/facilities/icare/. Accessed January 1 2022
- 16.Bottle A., Cohen C., Lucas A., et al. How an electronic health record became a real-world research resource: comparison between London's Whole Systems Integrated Care database and the Clinical Practice Research Datalink. BMC Med Inform Decis Mak. 2020;20:71. doi: 10.1186/s12911-020-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health England. Technical summary: public Health England data series on deaths in people with COVID-19. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908781/Technical_Summary_PHE_Data_Series_COVID-19_Deaths_20200812.pdf. Accessed January 1 2022
- 18.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.WHO. Global Equitable Access to COVID-19 Vaccines Estimated to Generate Economic Benefits of At Least US$ 153 Billion in 2020–21, and US$ 466 Billion by 2025, in 10 Major Economies, According to New Report by the Eurasia Group. https://www.who.int/news/item/03-12-2020-global-access-to-covid-19-vaccines-estimated-to-generate-economic-benefits-of-at-least-153-billion-in-2020-21. Accessed January 1, 2022
- 20.Matrajt L., Eaton J., Leung T., et al. Optimizing vaccine allocation for COVID-19 vaccines shows the potential role of single-dose vaccination. Nat Commun. 2021;12:3449. doi: 10.1038/s41467-021-23761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voysey M., Costa Clemens S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasileiou E., Simpson C.R., Shi T., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skowronski D.M., Setayeshgar S., Zou M., et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including alpha and gamma variants: a test-negative design in adults 70 years and older in British Columbia, Canada. Clin Infect Dis. 2021:ciab616. doi: 10.1093/cid/ciab616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J., Stoesser N., Matthews P.C., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6(9):1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monod M., Blenkinsop A., Xi X., et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371(6536):eabe8372. doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glampson B., Brittain J., Kaura A., et al. North West London Covid-19 vaccination programme: real-world evidence for vaccine uptake and effectiveness: retrospective cohort study. JMIR Public Health Surveill. 2021;7(9):e30010. doi: 10.2196/30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies N.G., Klepac P., Liu Y., Prem K., Jit M. CMMID COVID-19 working group, Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 29.Carazo S., Talbot D., Boulianne N., et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2 in healthcare workers extending 16 weeks post-vaccination: a test-negative design from Quebec, Canada. Clin Infect Dis. 2021:ciab739. doi: 10.1093/cid/ciab739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaillon A., Smith D.M. Phylogenetic analyses of SARS-CoV-2 B.1.1.7 lineage suggest a single origin followed by multiple exportation events versus convergent evolution. Clin Infect Dis. 2021:ciab265. doi: 10.1093/cid/ciab265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emary K.R.W., Golubchik T., Aley P.K., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders R.W., de Jong M.D. Pandemic moves and countermoves: vaccines and viral variants. Lancet. 2021;397:1326–1327. doi: 10.1016/S0140-6736(21)00730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh A., McMenamin J., Taylor B., Robertson C., Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baraniuk C. Covid-19: how effective are vaccines against the delta variant? BMJ. 2021;374:n1960. doi: 10.1136/bmj.n1960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.