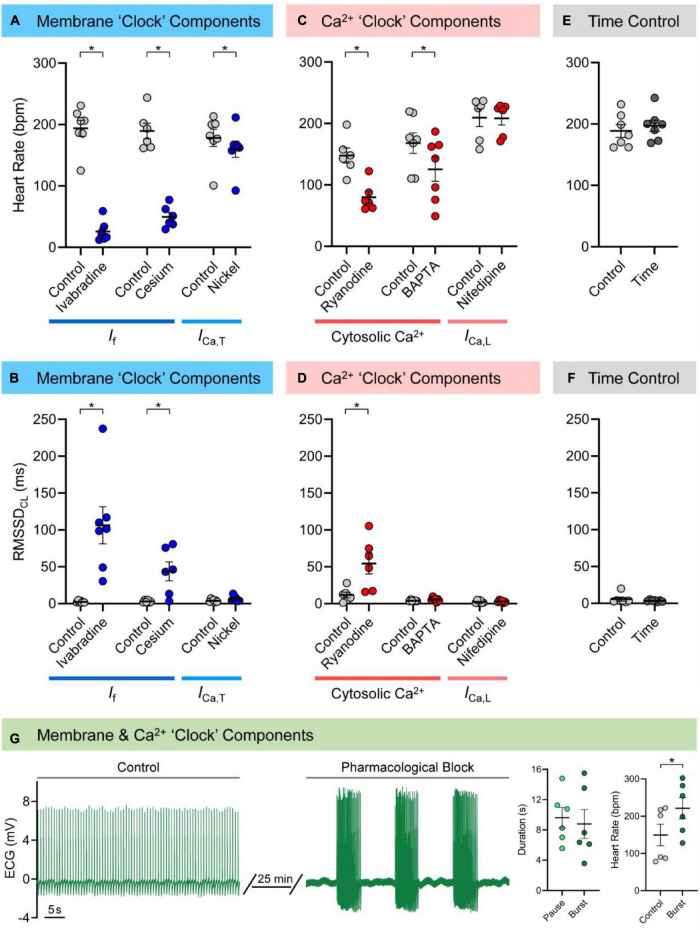
FIGURE 1.
Mechanisms of zebrafish sinoatrial node (SAN) automaticity. Effects of pharmacological block of membrane (40 min of 3 μM ivabradine hydrochloride for intracellular block of “funny” current, If, n = 7; 7 min of 3 mM cesium chloride for extracellular block of If, n = 6; 15 min of 165 μM nickel(II) chloride for block of T-type Ca2+ current, ICa,T, n = 7) or Ca2+ (15 min of 1 μM ryanodine for block of ryanodine receptors, n = 6; 30 min exposure of 5 μM BAPTA-AM, followed by 30 min to allow for de-esterification of the AM group, for chelation of cytosolic Ca2+, n = 7; 15 min of 0.2 μM nifedipine for block of L-type Ca2+ current, ICa,L, n = 6) “clock” components, as well as matched time controls (60 min, n = 7) on heart rate (A,C,E) and the inter-beat variability of cycle length (measured as the root mean square of its successive differences, RMSSDCL; B,D,F), in the isolated zebrafish heart. Representative electrocardiogram (ECG) recording of zebrafish cardiac rhythm in control and after combined pharmacological block of membrane and Ca2+ “clock” components (3 mM cesium chloride, 165 μM nickel(II) chloride, and 1 μM ryanodine, n = 6) and measurements of the resulting SAN pause and burst duration and control and burst heart rate (G). Data shown as mean ± SEM. Means compared by paired, two-tailed, Student’s t-tests; *p < 0.05.