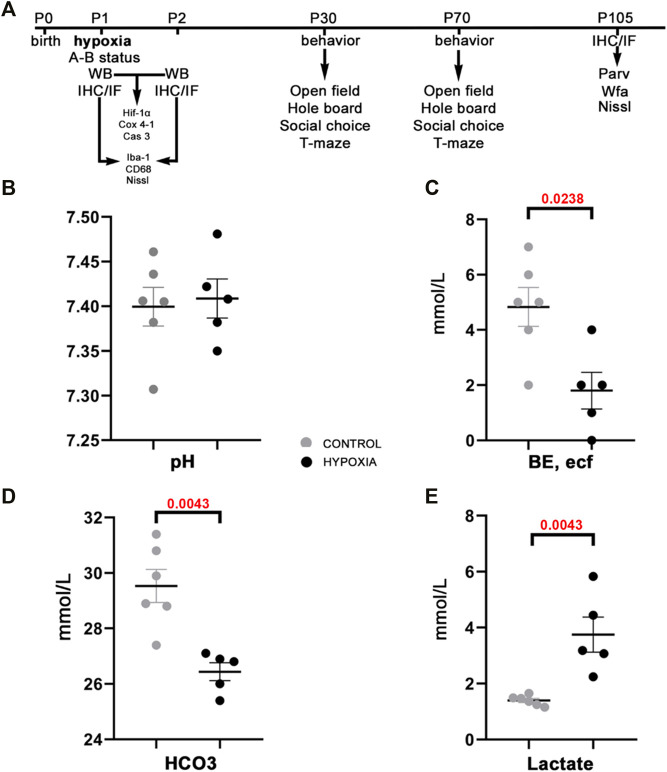
FIGURE 1.
The study design and acid-base status of the animals indicate sufficient compensatory buffers capacity, ensuring acid-base homeostasis at this design of moderate perinatal hypoxia in rat. (A) A presentation of the study design by timeline with the time points chosen for data acquisition by various tests, staining’s of brain tissue sections, and subsequent qualitative and quantitative analysis of the behavior and brain structures. Treated and control animal distribution per each experiment is presented in Supplementary Table S1. The treated rats were subjected to hypoxia (during 2 h) at P1. Immediately after, some were sacrificed to measure different parameters of A-B status. Other animals were sacrificed later at 8 h, and 24 h after hypoxia for brain samples, on which WB, IF, IHC were employed with different antibodies. The antibodies used in the study are listed in Table 1. The remainder of the animals were tested at two different age points: starting at P30 and P70 during five subsequent days. The animals were tested with four behavioral tests (open filed, hole board, social choice, T-maze). These animals were sacrificed at P105 to isolate the brain tissue for differential staining’s and further data acquisition. (B–E) The values of different blood parameters showing acid-base status measured immediately after hypoxia. (B) The pH (hydrogen potential) values speak in favor of sufficient compensatory capacity that ameliorates electrolyte imbalance in the rat neonates. (C) BE, ecf (base excess in the extracellular fluid) show significantly lower values in the hypoxic animals due to depletion of base buffers for compensation of the metabolic acidosis. (D,E) The HCO3 − (bicarbonate) and lactate concentrations in the blood show statistically significant differences between hypoxia-treated and control animals, proving shift from aerobic to anaerobic metabolic condition in the tissue as a consequence of hypoxia. All results are shown as mean ± standard error of the mean (SEM). P-postnatal day; A-B—acid-base status; WB-Western blot; IHC/IF—immunohistochemistry and/or immunofluorescence; Hif-1α—hypoxia-inducible factor 1α; Cox 4-1—cytochrome c oxidase subunit 4 isoform 1; Cas 3—caspase 3; Iba-1—ionized-calcium-binding-adaptor-molecule-1; CD68—class D scavenger receptor 68; Nissl (modification of cresyl-violet) staining; Parv—parvalbumin; Wfa—Wisteria floribunda agglutinin.