Figure 1.
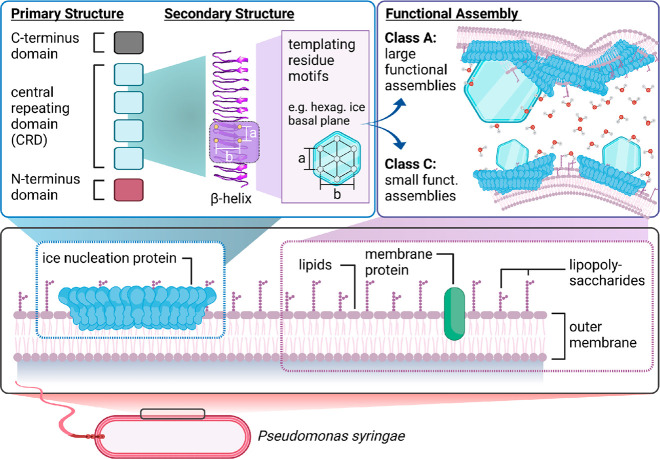
Overview of the proposed structure and working mechanism of bacterial ice nucleation proteins anchored to the outer cell membrane of P. syringae. The INP consists of an N-terminal, a C-terminal, and a central repeating domain. Their general function is to order water molecules into an “ice-like” arrangement to nucleate ice formation. This process is facilitated when INPs assemble into larger aggregates.