Abstract
Background
COVID-19 is an infectious disease of variable severity caused by a new coronavirus. Clinical presentation ranges from asymptomatic cases to severe illness. Most cases in newborns appear to be asymptomatic or mild.
Objective
To conduct a systematic review of the literature on published studies of COVID-19 in newborns with a positive RT–PCR test.
Methods
The PubMed and EMBASE databases were searched for infection data in newborns from 1 December 2019–21 May 2021. The mesh terms included “SARS-CoV-2”, “COVID-19”, “novel coronavirus”, “newborns” and “neonates”. The selection criteria were as follows: original studies reporting clinical, radiological, laboratory, and outcome data in newborns with a positive RT–PCR test for SARS-CoV-2. Two independent investigators reviewed the studies.
Results
Seventy-two studies that involved 236 newborns were included. The main clinical manifestations were fever (43.2%), respiratory (46.6%), and gastrointestinal (35.2%) symptoms; 60.1% had mild/moderate disease. A total of 52.5% had a chest X-ray; 43.5% were normal, and 24.1% reported consolidation/infiltration images. The most frequent laboratory abnormalities were elevated C reactive protein and elevated procalcitonin and lymphopenia. Mortality was 1.7%.
Conclusion
Symptoms of SARS-CoV-2 infection were mild to moderate in most of the newborns. The prognosis was good, and mortality was mainly associated with other comorbidities.
Key Words: SARS-CoV-2, COVID-19, Novel coronavirus, Newborn, Neonate, Systematic review
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the SARS-CoV-2 virus (severe acute respiratory syndrome–coronavirus-2), and since its original detection in Wuhan, Hubei Province, China, by the end of December 2019, it had become a rapidly growing global pandemic (1).
SARS-CoV-2 is a novel beta-coronavirus (1,2), and coronaviruses can mutate and recombine quickly, resulting in new viruses that can be transmitted from animals to humans (3).
SARS-CoV-2 is highly infectious and affects all susceptible populations, including children and newborns (4,5). Maternal and neonatal infections have been reported. Transmission of SARS-CoV-2 occurs mainly through direct or indirect contact with infected respiratory secretions or droplets, which are expelled when an infected person coughs, sneezes, or talks. Airborne transmission can also occur during procedures that generate aerosols, and indirect contact transmission through fomites is also possible (6., 7., 8.). To date, evidence supporting vertical transmission is controversial (7,9).
COVID-19 is primarily a respiratory disease, and its severity can range from asymptomatic to fatal cases (1). Infants, young children, and newborns have been reported to have a milder form of the disease than adults (4,5,10., 11., 12.). However, there is also evidence of pneumonia in neonatal early-onset SARS-CoV-2 infection (13). Reports of newborns with SARS-CoV-2 infection are still scarce and mainly consist of case series and case reports (14,15). Until May 2021, some systematic reviews have been published on COVID-19 in pregnant women and their perinatal outcomes. However, some problems can limit the validity of these reviews. For example, they include patients with suspected but not confirmed infection or with only one positive reverse transcriptase-polymerase chain reaction test (RT–PCR) close to the time of birth. Other reviews report maternal and neonatal data, or include all pediatric ages (11,16). A meta-analysis of COVID-19 in newborns includes cases with positive RT–PCR tests and/or the presence of specific IgM, including articles published between December 1, 2019, and August 2020 (17).
This study aimed to systematically review the literature regarding published studies on the main clinical, radiological, and laboratory characteristics, as well as the management and outcome of newborns with COVID-19 with a positive RT–PCR test.
Material and Methods
Search Strategy and Selection Criteria
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA). Databases including PubMed and Embase from December 1, 2019–May 21, 2021. There were no restrictions in publication language. The MesH terms used were “SARS-CoV-2”, “COVID-19”, “novel coronavirus”, “newborns” and “neonates”. Inclusion criteria were set as original studies, case series, case reports, and letters to the editor. Narrative and systematic revisions were excluded. References from the included studies and review articles were also analyzed to identify missing studies. An exhaustive review was conducted to avoid duplicate or overlapping cases.
In the included studies, newborns met the following criteria: positive SARS-CoV-2 RT–PCR test and clinical, radiological, laboratory, and patient outcome data. Newborns who had only one positive RT–PCR test at birth, without another confirmatory test, were eliminated to avoid including patients with possible transient colonization.
The titles and abstracts were reviewed by two independent researchers (AAL, PMR); disagreements between the two were resolved with a third investigator (HG).
Data Extraction and Quality Assessment
Data collected from full-text articles included study design, publication date, country, language, number of cases, maternal COVID-19, or SARS-CoV-2 infection, contact with suspected or confirmed cases, gestational age, birth weight, sex, diagnostic tests, neonatal specimen, age at diagnosis, age at onset of symptoms, comorbidity, clinical, radiological and laboratory data, treatment, and outcome.
The severity of the disease was evaluated according to the criteria established by Fang F, et al. and was grouped into 5 categories: asymptomatic, mild, moderate, severe, and critical (18).
Two independent reviewers used the tool Murad MH, et al. (19) proposed to evaluate the methodological quality of case reports and case series. The tool consists of four domains (selection, ascertainment, causality, and reporting) with eight questions to aid a quality score, and according to Della Gatta AN, et al. (20), a study was classified as “good quality” if all four domains were met, “intermediate quality” if three domains were met and “poor quality” if one or two domains were satisfied.
Data Analysis
A qualitative synthesis of the included studies was performed. Categorical variables are presented as frequencies and percentages, while continuous variables are expressed as medians and ranges. It should be noted that in many studies, not all data were registered.
The present research involved secondary use of published data. The IRB approved the study.
Results
With the search strategy, 1,587 articles were found in the databases, and after eliminating duplicates, 781 titles and abstracts were screened. Of the latter, 160 were selected for full-text review, but only 72 studies fulfilled the selection criteria (Figure 1 ). Of the 72 full-text articles included, 62 were case reports or case series, nine were cohort studies (13,21., 22., 23., 24., 25., 26., 27., 28.), and one was a cross-sectional study (29). In the 72 studies, 1,246 newborns were reported; however, 1,010 patients were excluded because 630 were born to COVID-19-positive mothers but had a negative RT–PCR test, 308 were infants, 69 did not underwent RT–PCR tests, and three newborns had only positive SARS-CoV-2 antibodies. Thus, this systematic review comprised data from 236 newborns. Supplementary Table 1 summarizes the data of the 72 included studies (13., 14., 15.,21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69., 70., 71., 72., 73., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87., 88., 89.).
Figure 1.
Flow diagram (PRISMA) of the study selection process.
General Characteristics of Newborns
All 236 newborns had positive SARS-CoV-2 RT–PCR tests, mainly performed in respiratory specimens: 48.6% (107/220) had nasopharyngeal swabs and 37.7% (83/220) had nasopharyngeal and oropharyngeal swabs. Other samples included nasal and respiratory secretions by tracheal aspiration or bronchoalveolar lavage, blood, stool, and anal swabs. In 16 newborns, specimen data was not specified.
The age of diagnosis ranged from 0 (day of birth) to 78 d, with a median of 10 d. The newborns with a positive RT–PCR test at birth were retested between 1 and 18 d, confirming the infection. Three patients had more than 28 d of postnatal life; however, these patients were premature, and at the time of diagnosis, two were 38 weeks, and one was 35 weeks of postconceptional age; therefore, they were also included.
One hundred and fifty-five newborns had a history of maternal COVID-19, most of which (92.9%) were confirmed by a positive RT–PCR test and suspected in the rest. In 65 newborns, data on maternal illness/infection were not specified. Fifteen newborns presented symptoms at birth; 13/15 were born to mothers with a positive RT–PCR test, 10/13 mothers were positive between 6 h and 16 d before delivery, and three at delivery time. Seven neonates presented symptoms in the first 24 h of life, six of their mothers had a positive RT–PCR test, and five of the six presented symptoms between one and two days before delivery.
In 99 of 173 neonates, there was a history of family members with COVID-19, including 49 confirmed by RT–PCR test and 50 suspected cases. In 63 neonates, data of infected contacts were not referred.
Seventy-four percent were newborns at full term (154/208) and 26% were premature. In 11.8% (n=28) of the cases, gestational age was not reported. Birth weight ranged from 900–4400 g (median 3120 g) in 116 neonates and was unknown in the rest. Of the 212 neonates for whom sex was reported, 123 (58%) were male.
Fifty-three neonates (22.4%) had some type of comorbidity, mainly prematurity; in nine (3.8%), the data were unknown.
Clinical Characteristics Associated with SARS-COV-2 Infection
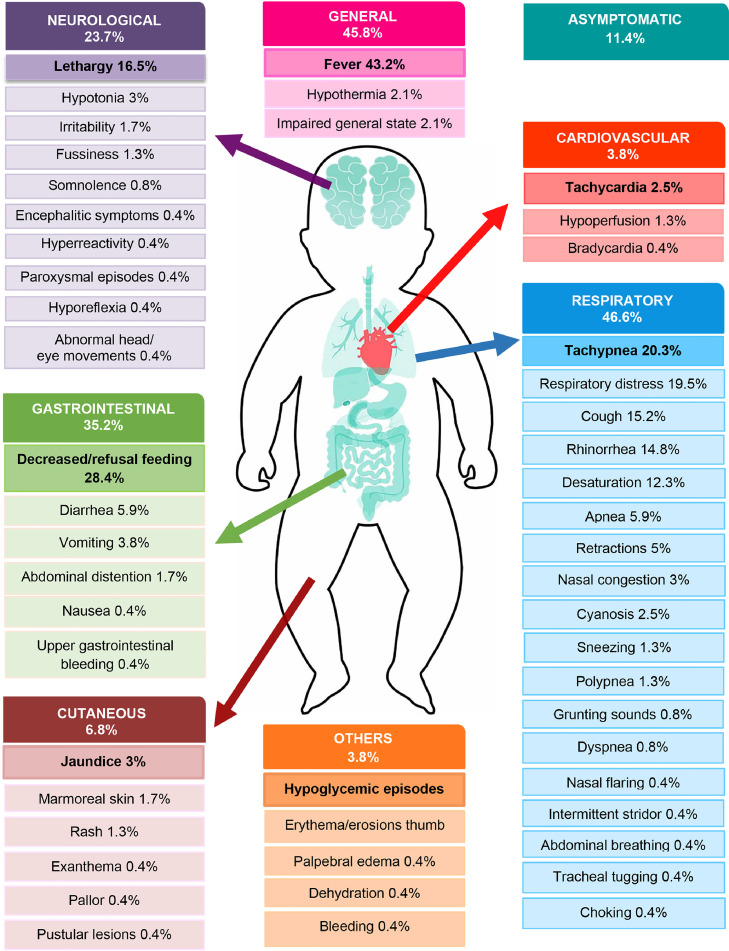
Summarized clinical data of 236 newborns are represented graphically in Figure 2 . The primary clinical manifestation was fever in 43.2%, which was followed by respiratory signs and symptoms in 46.6% (including respiratory distress, tachypnea, and cough). Gastrointestinal manifestations were reported in 35.2% of patients, such as decreased feeding and diarrhea. Neurological alterations were found in 23.7% of the neonates, mainly lethargy. Less frequent manifestations included cutaneous in 6.8% and cardiovascular in 3.8% (including tachycardia, hypotension, acidosis, and/or delayed capillary refill time).
Figure 2.
Clinical characteristics in 236 neonates with SARS-CoV-2 infection.
According to COVID-19 severity, 142 (60.2%) were classified as mild/moderate, 63 as severe (26.7%), 4 as critical (1.7%), and 27 (11.4%) neonates as asymptomatic.
Radiological Findings and Laboratory Tests
In 142 neonates, reports of radiological studies were available; 88% of them had a chest X-ray, of which 43.2% were normal. The main abnormal findings on radiographs were consolidation or infiltration images (24.0%) and ground-glass opacities (15.2%). Chest CT (computed tomography) was performed in 21 neonates, and ground-glass opacities were reported in nine neonates. Only 12 neonates had a lung ultrasound, and five of them reported signs of pulmonary edema of varying degrees Table 1. summarizes the findings on chest X-rays, chest CT, and lung ultrasound.
Table 1.
Radiological findings in 236 neonates with SARS-COV-2 infection
| n | % | |
|---|---|---|
| Not reported | 94 | 44.5 |
| Chest X-ray | 125/142 | 88.0 |
| Normal | 54 | 43.2 |
| Consolidation/infiltration | 30 | 24 |
| Bilateral ground glass opacities | 19 | 15.2 |
| Abnormal (not specified) | 7 | 5.6 |
| Bilateral diffuse opacification | 6 | 4.8 |
| Others | 9 | 7.2 |
| Chest computed tomography | 21 | 14.8 |
| Normal | 1 | 4.8 |
| Ground glass opacities | 9 | 19.0 |
| Bilateral pulmonary lesion | 3 | 14.2 |
| Others | 8 | 38.0 |
| Lung ultrasound | 12 | 8.4 |
| Normal | 1 | 8.3 |
| Signs of pulmonary edema of varying degrees | 5 | 41.7 |
| Coalescent B-lines and consolidation | 2 | 1.4 |
| Others | 4 | 33.3 |
Some children had more than one radiological study.
Twelve percent of the laboratory data were normal. The most frequent abnormal test was elevated C reactive protein in 16.6%, elevated procalcitonin in 11%, and lymphopenia in 9.4% Table 2. summarizes the laboratory findings.
Table 2.
Laboratory data in 236 neonates with SARS-CoV-2 infectiona
| n | % | |
|---|---|---|
| Not reported | 44 | 18.6 |
| Normal laboratory tests | 23/192 | 12.0 |
| Elevated C reactive protein | 32 | 16.6 |
| Elevated procalcitonin. | 21 | 11.0 |
| Lymphopenia | 18 | 9.4 |
| Neutropenia | 17 | 8.8 |
| Elevated lactate | 17 | 8.8 |
| Elevated liver function tests | 14 | 7.3 |
| Elevated D-dimer | 9 | 4.7 |
| Thrombocytopenia | 9 | 4.7 |
| Elevated interleukin (IL-6) | 6 | 3.1 |
| Leukopenia | 6 | 3.1 |
| Elevated creatine kinase | 5 | 2.6 |
| Elevated lactate dehydrogenase | 5 | 2.6 |
| Elevated lymphocytes | 2 | 1.0 |
| Others | 19 | 9.9 |
Abnormal values according to established parameters for newborns and local laboratories.
Management
Almost half of the newborns (48.2%) were admitted to a neonatal intensive care unit (NICU), while 26.6% were admitted to a pediatric ward and 6% to a neonatology ward. There was no information for 37 (15.6%) neonates regarding area of hospitalization. The median hospital stay was 12 d (range 1–69). Ten newborns were still hospitalized at the time of publication. In 31 (13.1%) newborns, the length of hospital stay was not reported.
Twenty-four neonates required invasive mechanical ventilation, 32 received noninvasive ventilation (nCPAP), and 61 were given supplemental oxygen by nasal cannula.
One hundred and four (46%) neonates received empirical antibiotics, and 45 (19.9%) received antiviral therapy (oseltamivir, acyclovir, remdesivir, lopinavir/ritonavir, hydroxychloroquine, and azithromycin). The use of immunomodulators (intravenous immunoglobulin, corticosteroids, and alpha interferon) was not frequent (5.3%). Only 54 (23.9%) newborns were breastfed (Table 3 ).
Table 3.
Management and care of 236 neonates with SARS-CoV-2 infection
| n | % | |
|---|---|---|
| Place of health care | ||
| Not referred | 37 | 15.6 |
| Neonatal Intensive Care Unit | 96/199 | 48.2 |
| Pediatric ward | 53 | 26.6 |
| Neonatology ward | 12 | 6.0 |
| Postnatal ward | 12 | 6.0 |
| Pediatric intensive care unit | 11 | 5.5 |
| Rooming-in | 5 | 2.5 |
| Home | 10 | 5.0 |
| Management | 226 | 95.8 |
| Support care/symptomatic treatment. | 85 | 37.6 |
| Isolation | 55 | 24.3 |
| Intravenous fluids | 9 | 3.9 |
| Resuscitation | 5 | 2.2 |
| Support | ||
| Respiratory support a | ||
| Conventional mechanical ventilation | 24 | 10.6 |
| Noninvasive ventilation (nCPAP) | 32 | 14.1 |
| Supplemental Oxygen | 61 | 27 |
| Drugs | ||
| Empirical antibiotics | 104 | 46.0 |
| Antivirals | 45 | 19.9 |
| Immunomodulators | 12 | 5.3 |
| Feeding (Breastmilk) | 54 | 23.9 |
One neonate required conventional mechanical ventilation and high-frequency ventilation, one required extracorporeal membrane oxygenation (ECMO) and one required inhaled nitric oxide. Twenty-one infants required more than one respiratory support modality
Outcome
Four deaths (1.7%) were reported. One newborn with Down syndrome and atrioventricular septal defect who developed methicillin-resistant Staphylococcus epidermidis sepsis and died after 21 d of hospitalization in a NICU due to neonatal acute respiratory distress syndrome; a premature infant of 900 g, 28 weeks of gestational age, died on the 7 d of life with respiratory failure and bleeding; another premature infant of 34 weeks gestational age, weight 2100 g with respiratory distress and pulmonary hemorrhage; and the final infant with a cause of death unrelated to SARS-CoV-2.
Supplementary Table 1 describes the clinical, radiological, laboratory, and management and outcome data of the 236 neonates included.
Quality Assessment of the Included Studies
Of the 62 articles evaluated using the Mayo Evidence-Based Practice Center tool for case reports and case series, fifteen (24.2%) were classified as good quality, 32 (51.6%) as intermediate quality, and fifteen (24.2%) as low quality.
Discussion
This systematic review describes information exclusively on COVID-19 in neonates with a confirmed infection by RT–PCR test, and, as far as we know, it is the first time that complete information of newborns with a positive confirmatory test is summarized. In five newborns, RT–PCR was performed at birth because their mothers had confirmed infection. Additional tests during the follow-up were also positive. In most of the patients (94.5%), the test was performed on a respiratory specimen.
In 72.4% of the newborns, there was a confirmed maternal history of SARS-CoV-2 infection, although the data related to possible acquisition times were not complete in all the cases. Vertical transmission has been questioned since the beginning of the pandemic. A recent systematic review that included 936 SARS-CoV-2 tested newborns born to pregnant women with COVID-19 infection found that maternal-to-fetal transmission of the virus might occur in approximately 3.2% during the third trimester (90). In our review, 21 newborns presented symptoms at birth or in the first 24 h, and most of their mothers had a confirmed infection, indicating acquisition of the virus in the period close to delivery. Infection during the first trimester has been even more challenging to confirm. As most infections were postnatally acquired, the importance of maintaining preventive measures during hospitalization and at home is emphasized.
This review, which included 236 newborns, found that only 11.4% were asymptomatic, in contrast with the systematic review by Raschetti R, et al. (17) that found 55.7% (97/176) asymptomatic cases, but like the review by Liguoro I, et al. that reported 20% of asymptomatic neonates but only included 25 cases (11). Most of the symptomatic cases presented a mild or moderate illness; however, 26.7% had severe illness, and 1.7% were critical, which is like the distribution among older children (11). Raschetti R, et al. did not mention the severity of the disease (17). The most common symptoms were comparable in the reviews, with respiratory registered as the main symptom, followed by general (fever), and finally gastrointestinal and neurological symptoms occurring at the same frequency (17). It is difficult to discern the role of SARS-CoV-2 infection during the neonatal period in clinical manifestations due to the patients’ comorbidities.
Approximately half of the neonates were admitted to the NICU (48.2%), and in addition to prematurity, underlying conditions, or symptomatic illness, hospitalization indications were isolation, observation, and monitoring. A significant proportion of neonates were successfully managed in neonatal or postnatal wards. The Centers for Disease Control and Prevention (CDC) of the U.S.A. recommends admission to the NICU for infants at higher risk for severe illness, such as preterm neonates and neonates with underlying medical conditions or those needing higher levels of care (91).
The hospital stay was variable, from 1–69 d (median 12), but in most cases, length of hospitalization was related to comorbidities, mainly involving premature newborns <35 weeks of gestational age and newborns with a severe or critical illness.
Several theories have been proposed to explain the mild expression of COVID-19 in children. One of them is that SARS-CoV-2 needs the presence of an angiotensin-converting enzyme 2 (ACE2) receptor to enter the cell; ACE2 receptors are expressed in the epithelium of the human airways and the pulmonary parenchyma. ACE2 is less mature in young children, so it may not function properly as a receptor for SARS-CoV-2; in addition, the ACE2-induced response in alveolar epithelium cells in children may be lower than that in adults (92., 93., 94.).
Another possible explanation is that in young children, the inflammatory response is less intense due to the immaturity of their immune system (95), but infection can also progress to moderate or severe disease. It is essential to have a reliable medical history of contacts and be aware of subtle symptoms and signs to provide adequate management and follow-up.
Findings on chest X-rays were like those reported in other ages (96,97). Raschetti R, et al. (17) reported that lung imaging was abnormal in 64% of cases, which is like the 56.8% reported in this review.
Laboratory data highlight the elevation of procalcitonin and C-reactive proteins. In the complete blood count, the most common alteration was lymphopenia. Even though these abnormalities are found in adult patients, although frequencies are much lower (98).
To date, there is no definitive recommendation for specific treatment of children with COVID-19, and it is not surprising that treatment provided to newborns was diverse. Intended antiviral treatment included oseltamivir, hydroxychloroquine, azithromycin, lopinavir/ritonavir, acyclovir, and remdesivir. These treatments are not approved for the management of COVID-19 pediatric patients. The FDA issued an Emergency Use Authorization (EUA) for remdesivir on May 1, 2020, to treat suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease (99,100). Nevertheless, a systematic review by Ansems K, et al. (101) found that based on available evidence, remdesivir probably has little or no effect on all-cause mortality at up to 28 d in hospitalized adults with COVID-19, and there is uncertainty about the effects of remdesivir on clinical improvement and mortality. There is no recommendation for its use in newborns.
Regarding feeding practices, only 23.9% of newborns were reported to be exclusively breastfed. At the beginning of the pandemic, some guidelines recommended discontinuing breastfeeding for babies born to mothers with proven or suspected COVID-19 (102). Currently, this recommendation has changed, and all babies must be breastfed, as no evidence demonstrates the transmission of infection from a mother to her child through breast milk. If the mother confirms the infection, she must wear a facial mask during breastfeeding and comply with preventive measures such as hand hygiene. If the newborn also has a positive RT–PCR test, health care personnel must wear personal protective equipment. At home, recommendations are similar when caring for a COVID-19 patient (103., 104., 105., 106.).
According to the analysis presented here, the prognosis of newborns with COVID-19 can be considered good, with a mortality rate of less than 2%. Only four deaths were reported, two in premature newborns, one in a patient with Down syndrome, congenital heart disease, and sepsis, and one of a cause not related to COVID-19. Ten newborns were still hospitalized at the time of publication of the case, so their outcome is not known, although to that moment, they were in good medical condition.
One of the study limitations is that most of the publications included in the review correspond to a level of evidence 4, according to the Oxford CEBM (Evidence-based Medicine Classification Center) (107). That means in the case reports and case series, only nine were retrospective cohort studies, and one was cross-sectional; in addition, 24.2% were of poor quality according to the tool used for evaluation. Many of the published articles focus on pregnant women with SARS-CoV-2 infection, and the characteristics of newborns are seldom described in detail. There is no follow-up of infected neonates, especially asymptomatic neonates, so we do not know if they subsequently developed symptoms or if patients with mild/moderate illness persist with symptoms or sequelae that appear later in life, like adult patients with long COVID (108).
Conclusions
Symptoms of COVID-19 in the neonatal period are mild. The mortality seems to be low, and it was associated with comorbidities. Neonates born to SARS-CoV-2-positive mothers should be followed up for timely detection of the disease and possible progression of the infection. As vaccination progresses and mothers are protected, fewer cases are expected to occur in newborns.
Conflicts of Interest
None of the authors have declared any potential conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arcmed.2022.03.001.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weston S, Frieman MB. COVID-19: Knowns, Unknowns, and Questions. mSphere. 2020;5:e00203–e00220. doi: 10.1128/mSphere.00203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. J Emerg Med. 2020;58:712–713. [Google Scholar]
- 6.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong L, Tian J, He S, et al. Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamouroux A, Attie-Bitach T, Martinovic, et al. Evidence for and against vertical transmission for Severe Acute Respiratory Syndrome Coronavirus 2. Am J Obstet Ginecol. 2020;223:91. doi: 10.1016/j.ajog.2020.04.039. e1–91.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawat M, Chandrasekharan P, Hicar MD, et al. COVID-19 in Newborns and Infants—Low Risk of Severe Disease: Silver Lining or Dark Cloud? Am J Perinatol. 2020;37:845–849. doi: 10.1055/s-0040-1710512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng L, Xia S, Yuan W, et al. Neonatal Early-Onset Infection with SARS-CoV-2 in 33 Neonates Born to Mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanburoglu MK, Tayman C, Oncel MY, et al. A Multicentered Study on Epidemiologic and Clinical Characteristics of 37 Neonates with Community-acquired COVID-19. Pediatr Infect Dis J. 2020;39:e297–e302. doi: 10.1097/INF.0000000000002862. [DOI] [PubMed] [Google Scholar]
- 15.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020;52:427–429. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490–501. doi: 10.1016/j.ejogrb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raschetti R, Vivanti AJ, Vauloup-Fellous C, et al. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang F, Chen Y, Zhao D, et al. Recommendations for the Diagnosis, Prevention, and Control of Coronavirus Disease-19 in Children-The Chinese Perspectives. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.553394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Gatta AN, Rizzo R, Pilu G, et al. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020;223:36–41. doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbero P, Mugüerza L, Herraiz I, et al. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J Matern Fetal Neonatal Med. 2020:1–7. doi: 10.1080/14767058.2020.1793320. [DOI] [PubMed] [Google Scholar]
- 22.Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. doi: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solís-García G, Gutiérrez-Vélez A, Pescador Chamorro I, et al. Epidemiology, management and risk of SARS-CoV-2 transmission in a cohort of newborns born to mothers diagnosed with COVID-19 infection. An Pediatr (Engl Ed) 2021;94:173–178. doi: 10.1016/j.anpede.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savasi VM, Parisi F, Patanè L, et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19) Obstet Gynecol. 2020;136:252–258. doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DA, Mohagheghi P, Beigi B, et al. Spectrum of neonatal COVID-19 in Iran: 19 infants with SARS-CoV-2 perinatal infections with varying test results, clinical findings, and outcomes. J Matern Fetal Neonatal Med. 2020;12:1–10. doi: 10.1080/14767058.2020.1797672. [DOI] [PubMed] [Google Scholar]
- 27.Ayed A, Embaireeg A, Benawadh A, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth. 2020;20:754. doi: 10.1186/s12884-020-03461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oncel MY, Akın IM, Kanburoglu MK, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur J Pediatr. 2021;180:733–742. doi: 10.1007/s00431-020-03767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao F, Chen B, Xiao T, et al. Children with SARS-CoV-2 infection during an epidemic in China (outside of Hubei province) Ann Transl Med. 2020;8:849. doi: 10.21037/atm-20-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Wang D, Chen GC, et al. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Díaz C, López-Maestro M, Moral-Pumarega MT, et al. First case of neonatal infection due to SARS-CoV-2 in Spain. An Pediatr. 2020;92:237–238. doi: 10.1016/j.anpedi.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng LK, Tao XW, Yuan WH, et al. First case of neonate with COVID-19 in China. Zhonghua Er Ke Za. 2020;58:279–280. doi: 10.3760/cma.j.cn112140-20200212-00081. Chinese. [DOI] [PubMed] [Google Scholar]
- 33.Salvatori G, De Rose DU, Concato C, et al. Managing COVID-19-Positive Maternal-Infant Dyads: An Italian Experience. Breastfeed Med. 2020;15:347–348. doi: 10.1089/bfm.2020.0095. [DOI] [PubMed] [Google Scholar]
- 34.Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon M, Kagalwala T, Rezk K, et al. Rapid systematic review of neonatal COVID-19 including a case of presumed vertical transmission. BMJ Paediatrics Open. 2020;4 doi: 10.1136/bmjpo-2020-000718. e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumpa V, Kamity R, Vinci AN, et al. Neonatal Coronavirus 2019 (COVID-19) Infection: A Case Report and Review of Literature. Cureus. 2020;12:e8165. doi: 10.7759/cureus.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng XY, Tao XW, Zeng LK, et al. Application of pulmonary ultrasound in the diagnosis of COVID-19 pneumonia in neonates. Zhonghua Er Ke Za Zhi. 2020;58:347–350. doi: 10.3760/cma.j.cn112140-20200228-00154. Chinese. [DOI] [PubMed] [Google Scholar]
- 38.Coronado-Munoz A, Nawaratne U, McMann D, et al. Late-Onset Neonatal Sepsis in a Patient with COVID-19. N Engl J Med. 2020;382:e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piersigilli F, Carkeek K, Hocq C, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health. 2020;4:476–478. doi: 10.1016/S2352-4642(20)30140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–E650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang ZJ, Yu XJ, Fu T, et al. Novel coronavirus infection in newborn babies aged <28 d in China. Eur Respir J. 2020;55 doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonsenso D, Costa S, Sanguinetti M, et al. Neonatal Late Onset Infection with Severe Acute Respiratory Syndrome Coronavirus 2. Am J Perinatol. 2020;37:869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chacón-Aguilar R, Osorio-Cámara JM, Sanjurjo-Jiménez I, et al. COVID-19: Fever syndrome and neurological symptoms in a neonate. An Pediatría (English Ed) 2020;92:373–374. doi: 10.1016/j.anpede.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alzamora MC, Paredes T, Cáceres D, et al. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am J Perinatol. 2020;37:861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibarra-Ríos D, Villanueva-García D, Vázquez-Solano EP, et al. Lung ultrasound and neonatal COVID-19 pneumonia: A case report. Res Square. 2020 doi: 10.21203/rs.3.rs-33182/v2. [DOI] [Google Scholar]
- 46.Baquero H, Venegas ME, Velandia L, et al. Sepsis neonatal tardía por SARS CoV-2. Biomédica. 2020;40(2):44–49. doi: 10.7705/biomedica.5609. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvalho WB, Gibelli MAC, Krebs VLJ, et al. Neonatal SARS-COV-2 infection. Clinics (Sao Paulo) 2020;75:e1996. doi: 10.6061/clinics/2020/e1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregorio-Hernández R, Escobar-Izquierdo AB, Cobas-Pazos J, et al. Point-of-care lung ultrasound in three neonates with COVID-19. Eur J Pediatr. 2020;179:1279–1285. doi: 10.1007/s00431-020-03706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Precit MR, Yee R, Anand V, et al. A Case Report of Neonatal Acute Respiratory Failure Due to Severe Acute Respiratory Syndrome Coronavirus-2. J Pediatr Infect Dis Soc. 2020;13:390–392. doi: 10.1093/jpids/piaa064. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mithal LB, Machut KZ, Muller WJ, et al. SARS-CoV-2 Infection in Infants Less than 90 Days Old. J Pediatr. 2020;224:150–152. doi: 10.1016/j.jpeds.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X, Gao J, Luo X, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vertical Transmission in Neonates Born to Mothers with Coronavirus Disease 2019 (COVID-19) Pneumonia. Obstet Gynecol. 2020;136:65–67. doi: 10.1097/AOG.0000000000003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng KF, Bandi S, Bird PW, et al. COVID-19 in Neonates and Infants: Progression and Recovery. Pediatr Infect Dis J. 2020;39:e140–e142. doi: 10.1097/INF.0000000000002738. [DOI] [PubMed] [Google Scholar]
- 53.Feld L, Belfer J, Kabra R, et al. A case series of the 2019 novel coronavirus (SARS-CoV-2) in 3 febrile infants in New York. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1056. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Zhang Q, Zhao L, et al. Clinical and Imaging Features of COVID-19 in a Neonate. Chest. 2020;158:e5–e7. doi: 10.1016/j.chest.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinelli M, Paterlini G, Citterio M, et al. Early neonatal SARS-CoV-2 infection manifesting with hypoxemia requiring respiratory support. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1121. [DOI] [PubMed] [Google Scholar]
- 56.Venturini E, Palmas G, Montagnani C, et al. Severe neutropenia in infants with severe acute respiratory syndrome caused by the novel coronavirus 2019 infection. J Pediatr. 2020;222:259–261. doi: 10.1016/j.jpeds.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meslin P, Guiomard C, Chouakria M, et al. Coronavirus Disease 2019 in Newborns and Very Young Infants: a Series of Six Patients in France. Pediatr Infect Dis J. 2020;39:e145–e147. doi: 10.1097/INF.0000000000002743. [DOI] [PubMed] [Google Scholar]
- 58.Cook J, Harman K, Zoica B, et al. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc Health. 2020;4:548–551. doi: 10.1016/S2352-4642(20)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eghbalian F, Esfahani AM, Jenabi E. COVID-19 Virus in a 6-Day-Old Girl Neonate: A Case Report. Clin Pediatr (Phila) 2020;59:1288–1289. doi: 10.1177/0009922820946010. [DOI] [PubMed] [Google Scholar]
- 60.Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.04.030. 109.e1–109.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Guo L, Chen L, et al. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu YT, Liu J, Xu JJ, et al. Neonatal outcome in 29 pregnant women with COVID-19: A retrospective study in Wuhan, China. PLOS Med. 2020;17 doi: 10.1371/journal.pmed.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sola A, Rodríguez S, Cardetti M, et al. COVID-19 perinatal en América Latina. Rev Panam Salud Pública. 2020;44:e47. doi: 10.26633/RPSP.2020.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Govind A, Essien S, Karthikeyan A, et al. Re: Novel Coronavirus COVID-19 in late pregnancy: Outcomes of first nine cases in an inner-city London hospital. Eur J Obstet Gynecol Reprod Biol. 2020;251:272–274. doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun M, Xu G, Yang Y, et al. Evidence of mother-to-newborn infection with COVID-19. Br J Anaesth. 2020;125:e245–e247. doi: 10.1016/j.bja.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abasse S, Essabar L, Costin T, et al. Neonatal COVID-19 Pneumonia: Report of the First Case in a Preterm Neonate in Mayotte, an Overseas Department of France. Children (Basel) 2020;7:87. doi: 10.3390/children7080087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulkarni R, Rajput U, Dawre R, et al. Early-onset symptomatic neonatal COVID-19 infection with high probability of vertical transmission. Infection. 2021;49:339–343. doi: 10.1007/s15010-020-01493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenz N, Treptow A, Schmidt S, et al. Neonatal Early-Onset Infection With SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39:e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 72.Needleman JS, Hanson AE. COVID-19-associated apnea and circumoral cyanosis in a 3-week-old. BMC Pediatr. 2020;20:382. doi: 10.1186/s12887-020-02282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dima M, Enatescu I, Craina M, et al. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania. Medicine (Baltimore) 2020;99:e21284. doi: 10.1097/MD.0000000000021284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demirjian A, Singh C, Tebruegge M, et al. Probable Vertical Transmission of SARS-CoV-2 Infection. Pediatr Infect Dis J. 2020;39:e257–e260. doi: 10.1097/INF.0000000000002821. [DOI] [PubMed] [Google Scholar]
- 75.Patek P, Corcoran J, Adams L, et al. SARS-CoV-2 Infection in a 2-Week-Old Male With Neutropenia. Clin Pediatr (Phila) 2020;59:918–920. doi: 10.1177/0009922820920014. [DOI] [PubMed] [Google Scholar]
- 76.McLaren SH, Dayan PS, Fenster DB, et al. Novel coronavirus infection in febrile infants aged 60 days and younger. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1550. [DOI] [PubMed] [Google Scholar]
- 77.González-Brabin A, Iglesias-Bouzas MI, Nieto-Moro M, et al. Neonatal apnea as initial manifestation of SARS-CoV-2 infection. An Pediatr (Engl ed) 2020;93:215–216. doi: 10.1016/j.anpedi.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marzollo R, Aversa S, Prefumo F, et al. Possible Coronavirus Disease 2019 Pandemic and Pregnancy: Vertical Transmission Is Not Excluded. Pediatr Infect Dis J. 2020;39:e261–e262. doi: 10.1097/INF.0000000000002816. [DOI] [PubMed] [Google Scholar]
- 79.Siddhi PS, Rayasandra G, Plant AJ, et al. COVID-19 in a Preterm- Leading to Remodelling of Care. Indian J Pediatr. 2020;87:759. doi: 10.1007/s12098-020-03426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sisman J, Jaleel MA, Moreno W, et al. Intrauterine Transmission of SARS-COV-2 Infection in a Preterm Infant. Pediatr Infect Dis J. 2020;39:e265–e267. doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 81.Sagheb S, Lamsehchi A, Jafary M, et al. Two seriously ill neonates born to mothers with COVID-19 pneumonia- a case report. Ital J Pediatr. 2020;46:137. doi: 10.1186/s13052-020-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salik I, Mehta B. Tetralogy of Fallot palliation in a COVID-19 positive neonate. J Clin Anesth. 2020;66 doi: 10.1016/j.jclinane.2020.109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han MS, Seong MW, Heo EY, et al. Sequential Analysis of Viral Load in a Neonate and Her Mother Infected With Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hinojosa-Velasco A, de Oca PVB, García-Sosa LE, et al. A case report of newborn infant with severe COVID-19 in Mexico: Detection of SARS-CoV-2 in human breast milk and stool. Int J Infect Dis. 2020;100:21–24. doi: 10.1016/j.ijid.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wardell H, Campbell J I, VanderPluym C, et al. SARS-CoV-2 infection in febrile neonates. J Pediatr Infect Dis Soc. 2020;9:630–635. doi: 10.1093/jpids/piaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paret M, Lighter J, Pellett Madan R, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Febrile Infants Without Respiratory Distress. Clin Infect Dis. 2020;71:2243–2245. doi: 10.1093/cid/ciaa452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamaniyan M, Ebadi A, Aghajanpoor S, et al. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020;40:1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaytán-Flores B, Hernández-Moreno A, Jiménez-Sánchez C, et al. Presentación de un caso de un recién nacido prematuro con COVID-19. Educación y Salud. 2020;9:1–4. [Google Scholar]
- 89.Lima-Rogel V, Villegas-Silva R, Coronado-Zarco A, et al. Perinatal COVID-19: a case report, literature review, and proposal of a national system for case record. Bol Med Hosp Infant Mex. 2021;78:34–40. doi: 10.24875/BMHIM.20000230. [DOI] [PubMed] [Google Scholar]
- 90.Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gyneco. 2021;224:35–53. doi: 10.1016/j.ajog.2020.07.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CDC. Evaluation and Management Considerations for Neonates at Risk for COVID-19. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-newborns.html (Accessed July 16, 2021)
- 92.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depends on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Basha S, Surendran N, Pichichero M. Immune Responses in Neonates. Expert Rev Clin Immunol. 2014;10:1171–1184. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng MY, Lee EYP, Yang J, et al. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong HYF, Lam HYS, Fong AH, et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng X, Li S, Sun Q, et al. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coronavirus (COVID-19) 2020. Update: Daily Roundup. May 1Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-may-1-2020 (Accessed July 20, 2021) [Google Scholar]
- 100.NIH. COVID-19 Treatment Guidelines. Therapeutic Management of Hospitalized Adults With COVID-19; [last Updated: July 8, 2021 ]. Available at https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults-therapeutic-management/. (Accessed July 19, 2021).
- 101.National Institutes of Health . Therapeutic Management of Hospitalized Adults With COVID-19; 2022. COVID-19 Treatment Guidelines. Clinical management. Available at https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults-therapeutic-management/. (Accessed February 22, 2022) [Google Scholar]
- 102.Wang L, Shi Y, Xiao T, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann Transl Med. 2020;8:47. doi: 10.21037/atm.2020.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.World Health Organization. (2020). Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. World Health Organization. Available at https://apps.who.int/iris/handle/10665/330893 (Accessed August 2, 2021).
- 104.American Academy of Pediatrics. FAQs: management of infants born to mothers with suspected or confirmed COVID-19. [Last Updated 05/04/2021] Available at https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/. (Accessed August 10, 2021).
- 105.Centers for Disease Control and Prevention. Care for Breastfeeding People. Interim Guidance on Breastfeeding and Breast Milk Feeds in the Context of COVID-19. [Updated June 17, 2021 ] Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-women.html. (Accessed August 5, 2021).
- 106.UNICEF. Breastfeeding safely during the COVID-19 pandemic. How to nourish your child following the latest expert guidance. [last updated on 29 July 2021 ]. Available at https://www.unicef.org/coronavirus/breastfeeding-safely-during-covid-19-pandemic (Accessed August 2, 2021).
- 107.CEBM . 2009. Oxford Centre for Evidence-Based Medicine: Levels of Evidence. MarchAvailable at https://www.cebm.net/2009/06/oxford-centre-evidence-basedmedicine-levels-evidence-march-2009/ (Accessed March 20, 2021) [Google Scholar]
- 108.Yan Z, Yang M, Lai CL. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines. 2021;9:966. doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.