Abstract
Oral antigen administration to induce regulatory T cells (Treg) takes advantage of regulatory mechanisms that the gastrointestinal tract utilizes to promote unresponsiveness against food antigens or commensal microorganisms. Recently, antigen-based oral immunotherapies (OITs) have shown efficacy as treatment for food allergy and autoimmune diseases. Similarly, OITs appear to prevent anti-drug antibody responses in replacement therapy for genetic diseases. Intestinal epithelial cells and microbiota possibly condition dendritic cells (DC) toward a tolerogenic phenotype that induces Treg via expression of several mediators, e.g. IL-10, transforming growth factor-β, retinoic acid. Several factors, such as metabolites derived from microbiota or diet, impact the stability and expansion of these induced Treg, which include, but are not limited to, FoxP3+ Treg, LAP+ Treg, and/or Tr1 cells. Here, we review various orally induced Treg, their plasticity and cooperation between the Treg subsets, as well as underlying mechanisms controlling their induction and role in oral tolerance.
Keywords: Oral tolerance, Oral Immunotherapy (OIT), Regulatory T cell, LAP Treg, Tr1, Intestinal Immune System
Graphical Abstract
1. Introduction
The small and large intestine are continuously exposed to a large variety of foreign antigens derived from food as well as commensal bacteria. Nevertheless, the intestinal immune system does not necessarily mount cellular or humoral immune responses to non-self-antigens due to regulatory mechanisms. One such mechanism is termed “oral tolerance”, referring to the natural development of induced tolerance to orally ingested stimuli in the gut-associated lymphoid tissue (GALT) [1]. Failure to establish oral tolerance leads to food allergy or the development of intestinal inflammatory diseases [2, 3]. While the colon is thought to respond to the massive saturation stimulation by bacterial or bacterially induced antigens, the immune system of the small intestine is distinct; it processes orally delivered antigens as well as systemic antigens, contains specialized lymphoid structures such as Peyer’s patches, has a distinct microbiome, distinct mechanisms of immune regulation, and uniquely interacts with the mesenteric lymph node (MLN) [4–10]. Hence oral tolerance mechanisms, including oral induction of regulatory T cells (Treg), take place in the small intestine.
Recently, the term oral immuno-therapy (OIT) was coined to define the oral delivery of antigen with the objective to suppress immune responses. Delivering a specific target antigen or antibody that affects T cell function have been the two main methods used to achieve OIT. Alternatives routes of OIT include nasal, sublingual, subcutaneous and epicutaneous administration [11–15].
Oral tolerance was first demonstrated by Wells and Osbourne more than a century ago [16]. These investigators found that guinea pigs fed with corn-containing diet, but not corn-free diet, failed to show anaphylactic reactions against zein, a major protein of corn.
In 1946, another study reported that prior feeding of certain allergenic compounds to non-sensitive subjects induced a state of immunological tolerance against subsequent experimental dermal sensitization with the same compounds [17]. Subsequent studies confirmed the existence of an oral tolerance mechanism. For instance, rats developed tolerance to horse serum or pollen extract when fed with these antigen prior to non-oral exposure [18]. This was also demonstrated in other animal models that were fed bovine serum albumin [19] or sheep red blood cells [20]. However, induction of oral tolerance in humans was only demonstrated in the early 1990s, when adults fed with keyhole limpet hemocyanin followed by subcutaneous immunization with the same antigen were prevented from developing a subsequent delayed type hypersensitivity response [21]. Currently, OIT has been applied toward tolerance induction in autoimmunity and inflammatory diseases such as peanut allergy [22, 23], allergic asthma [24, 25], pollen allergy [26], hepatitis C infection [27], nonalcoholic steatohepatitis (NASH) [28], Pompe disease [29], rheumatoid arthritis [30, 31], type I diabetes [32, 33], hemophilia A and B [34–37] in clinical as well as preclinical studies.
The development of oral tolerance is thought to take place in both the small and large gastrointestinal (GI) tract, where the GALT plays a key role in regulating responses to ingested antigens [38]. Antigen can be acquired directly by phagocytes or can be delivered through goblet cell associated passages prior to capture by dendritic cells (DCs) in lamina propria (LP) [39]. Antigen uptake by a subset of regulatory DCs expressing CD103, which migrate from the gut mucosa to the MLN, concomitant secretory IgA production [40], forkhead box protein P3 (FoxP3) expressing Treg that produce transforming growth factor β (TGF-β) and IL-10, and expression of indoleamine 2,3-dioxygenase (IDO) or the vitamin A metabolite retinoic acid (RA) [41] are all implicated in this process.
For the last three decades, the role of T cells in oral tolerance induction has been studied in increasing detail. It was found that depletion of CD4+ T cells abolished oral tolerance development [42], and that oral tolerance could be transferred from one animal to another by adoptive CD4+ T cell transfer [43]. Moreover, a population of TGF-β secreting CD4+ T cells termed Th3 were found to play a key role in oral tolerance [44], supporting the role of Treg in the induction of oral tolerance. Another conceptual advance in the oral tolerance field was the discovery of CD4+ T cells expressing a membrane-bound form of TGF-β that contains latency-associated peptide (LAP) [1, 45, 46]. LAP+CD4+ T cells were found to have important immune regulatory functions in oral tolerance. Interestingly, TGF-β is required for the induction of both FoxP3+ Treg and LAP+ Treg [47, 48].
Here we review current understanding of oral tolerance mechanisms, applications of OIT in allergy, autoimmune disease and in inducing tolerance to protein replacement therapies for monogenic disorders. We highlight key regulatory cells that are induced by orally delivered antigen, circumstances leading to their induction and the suppressive mechanisms exerted by them. Understanding these mechanisms are critical to identify new strategies for modulating tolerance.
2. Subsets of orally induced regulatory T cells
In the intestine, crosstalk among several cells occurs in order to induce a naturally tolerogenic environment. Unlike thymus derived (t)Treg in other organs, which have a self-antigen TCR repertoire, intestinal Treg display a peripheral (pTreg) TCR repertoire responsive to resident and non-resident microbiota and dietary antigens, thus playing a crucial role in controlling pro-inflammatory responses [49–52]. Both tTreg and pTreg are located in the intestine, but it appears that the intestinal environment prefers the latter [49, 50], with dietary antigens driving the vast majority of small intestinal pTreg induction [53]. In accordance, pTreg, but not tTreg, are required for oral tolerance, as demonstrated by the development of oral tolerance even in the absence of thymus-derived Treg [54]. Development of orally induced tolerance includes diverse pTreg subsets such as FoxP3+ Treg and FoxP3− Treg (Figure 1 and Table 1). Both cell types, however, exhibit a certain degree of functional plasticity to adapt to a specific microenvironment, with overlapping function and cooperation, thus giving rise to each other in order to establish tolerance. Not only do FoxP3+ Treg display remarkable heterogeneity in expression of phenotypic and molecular signatures [55], they may lose FoxP3 expression with concurrent loss of suppressive function [56]. FoxP3− cells may also transiently express FoxP3 during activation [57].
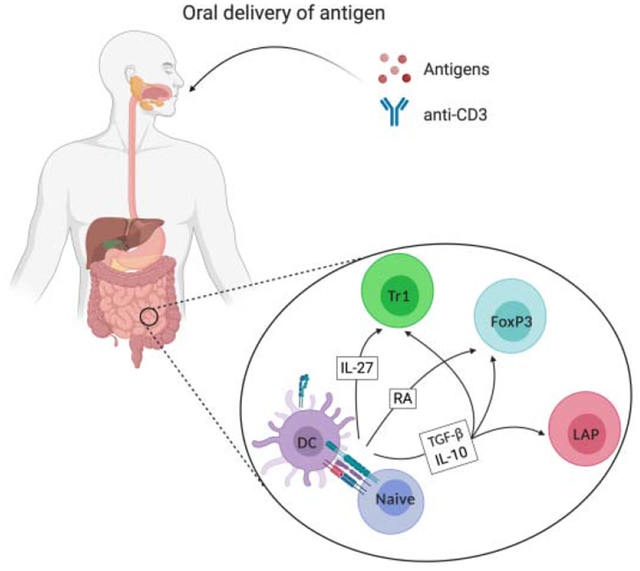
Figure 1: Types of orally induced CD4+ regulatory T cells.
Interactions of tolerogenic DCs in the MLN or in PP with naive T cells may result in the oral induction of three major types of CD4+ regulatory T cells: FoxP3+ Treg, LAP+ Treg and Tr1 cells. TGF-β and RA drive differentiation of FoxP3+ pTreg, which express TCR, CD4, CD25, FoxP3, GITR and CTLA-4, among other markers. FoxP3+ Treg produce suppressive cytokines such as TGF-β, IL-10, IL-35 as well as granzyme, which are critical to suppression of immune responses in OIT. Induction of LAP+ Treg is not completely understood, although TGF-β plays a key role. LAP+ Treg express TCR, CD4 and LAP, among other markers, on the surface. These cells secrete TGF-β as wells as IL-10, which are responsible for immunosuppressive proprieties. Tr1 cells express TCR, CD4, CD49b, LAG-3, among other markers, on the cell surface, and c-Maf and AhR transcription factors. Tr1 cell differentiation depends on IL-27 secreted by DCs and are characterized by high IL-10 secretion, but also secrete TGF-β.
Table 1.
Summary of types of regulatory T cells induced during OIT
| FoxP3+ | LAP+ | Tr1 | |
|---|---|---|---|
| Classical Inductors | TGF-β | TGF-β | TGF-β |
| RA | IL-27 | ||
| IDO | |||
| Transcription factors | FoxP3 | cMaf | |
| AhR | |||
| Extracell markers | CD25 | LAP | LAG-3 |
| CTLA4 | CD49 | ||
| GITR | |||
| Cytokines produced | TGF-β | TGF-β | TGF-β |
| IL-10 | IL-10 | IL-10 | |
| IL-35 | IL-21 | ||
| Granzyme | |||
| OIT inductors | Dietary antigens | Vitamin A | IL-2 (s.c.) |
| Vitamin A | Microbiota | Anti-CD3 (Nasal) | |
| Vitamin B9 | Anti-CD3 (Nasal or oral) | ||
| Microbiota | |||
| Low dose of IL-2 (i.p. or oral) | FVIII or FIX encapsulated in chloroplasts | ||
| FVIII encapsulated in chloroplasts |
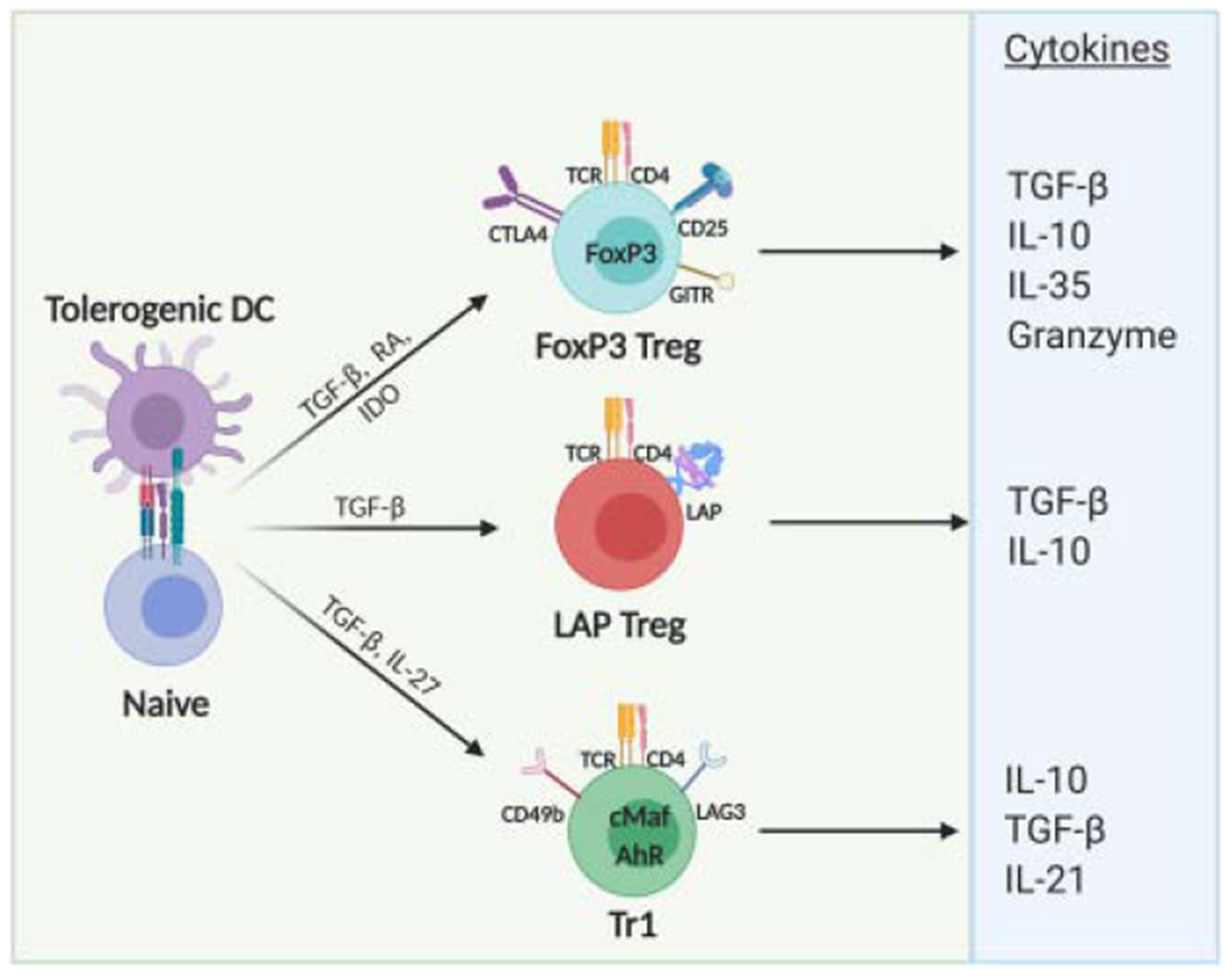
2.1. FoxP3+ Treg
The transcription factor FoxP3 is crucial to the development, maintenance and function of CD4+CD25+ Treg. Mutations in FoxP3 are associated with immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) in humans [58] and fatal autoimmunity in scurfy mice, which lack the FoxP3 gene [59]. Differentiation of naive T cells toward FoxP3+ Treg depends, among other factors, on TGF-β and RA [60–62]. RA is able to induce FoxP3 by binding to the nuclear RA receptors (RAR) and the retinoid X receptor (RXR), which function as transcription factors, binding to RA response elements (RAREs) to regulate gene expression [63–66]. A combination of SMAD3 phosphorylation and inhibition of IL-6 receptor expression (and other putative mechanisms) leads to enhanced TGF-β-induced Foxp3 expression and Treg conversion [66]. Conversely, in the presence of IL-6 and TGF-β caused by inflammatory conditions, naive T cells differentiate into a Th17 phenotype [67]. Thus, integration of TGF-β and other cytokine signals determine the outcome of CD4+ T cell differentiation. How TGF-β drives differentiation into LAP+ Treg (discussed below) as opposed to FoxP3+ Treg is less clear. Characteristics of FoxP3+ Treg include expression of the co-inhibitory receptor cytotoxic T lymphocyte antigen 4 (CTLA4), inducible T cell co-stimulator (ICOS), glucocorticoid-induced tumor necrosis factor receptor family related gene (GITR), low levels of CD45RB, integrin α4β7, αvβ8, glycoprotein A repetitions predominant (GARP), production of anti-inflammatory cytokines such as IL-10, TGFβ and IL-35, as well as regulation by IL-2 and ATP [68] (Figure 1).
FoxP3+ Treg are present in every organ of the body, constituting 5–10% of peripheral CD4+ T cells in mice and human [69]. However, these cells may represent a much higher proportion in the intestine. For instance, FoxP3+ cells represent approximately 30% of CD4+ T cells in the colonic LP and 20% in the LP of the small intestine [70, 71]. The role of FoxP3+ Treg in maintenance of intestinal homeostasis was demonstrated by Sakaguchi and colleagues, who observed the development of spontaneous autoimmune disease, including gastritis, when the CD25+ subpopulation of CD4+ T cells was depleted [72]. In line with these results, Powrie et al showed that transfer of CD4+CD25+ T cells in mice with colitis prevented intestinal inflammation [73], which was confirmed by other studies [74, 75]. Importantly, failure to generate FoxP3+ Treg in the gut-draining lymph nodes abrogates oral tolerance [76].
Because modulation of FoxP3 expression occurs at transcriptional, epigenetic, and posttranslational levels, FoxP3+ Treg show broad heterogeneity [77]. FoxP3 expression is regulated by intronic enhancers: non-coding sequences 1 to 3 (CNS1-CNS3) that recruit proteins to the FoxP3 locus. Studies in Treg have shown that TGF-β is able to trigger FoxP3 induction in pTreg through CNS1, although this may not assure full Treg function [64, 78]. CNS1-deficient mice have dysbiotic microbiota and enhanced Th2-type inflammatory response in the colon [79, 80]. CNS3 regulates the expression of FoxP3 in tTreg [64]. To elicit the full Treg signature, additional genes besides FoxP3 are required, such as IKAROS family zinc finger 4 (IKZF4), interferon regulatory factor 4 (IRF4), SATB homeobox 1 (SATB1), lymphoid enhancer binding factor 1 (LEF1), and GATA binding protein 3 (GATA3) [81]. FoxP3 expression may be lost under inflammatory conditions, resulting in a loss of suppressive function. These cells can actively contribute to inflammation through secretion of pro-inflammatory cytokines such as IFN-γ and IL-2 [82–84]. Such “ex-FoxP3” cells can acquire a Th17 phenotype [83, 85, 86] or convert into Th2-like cells in a IL-4 dependent manner in the gut [87]. Altogether, these reports suggest that induced FoxP3 Treg do not necessarily exhibit lineage stability (Figure 2).
Figure 2: Overlapping function, cooperative interaction and plasticity among cell types during establishment of immune regulation upon oral antigen delivery.
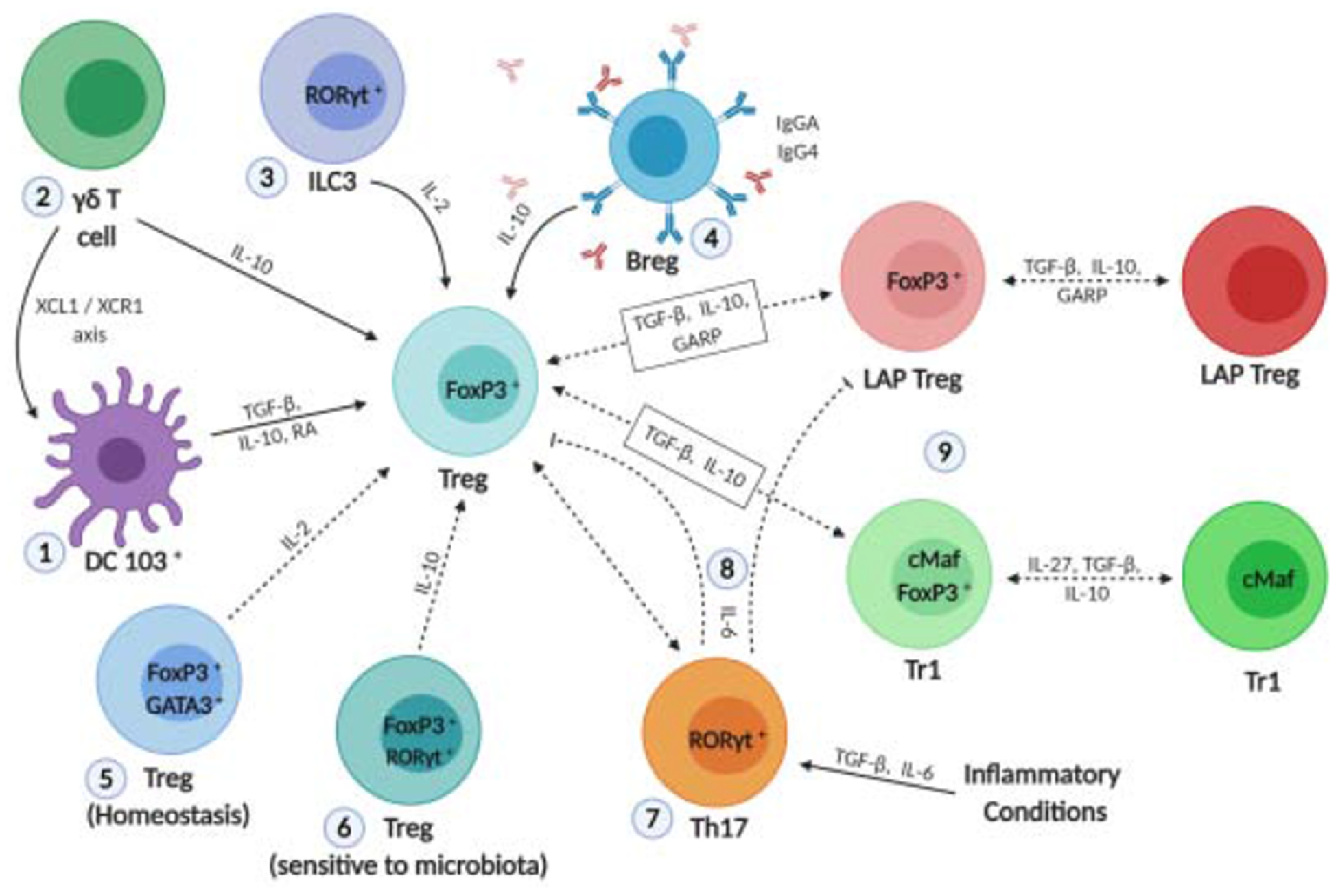
The success of OIT depends on crosstalk between several cell types in the GALT. 1) CD103+ DCs mediate differentiation of LAP+ Treg and produce RA, which in combination with TGF-β and IL-10 induces FoxP3+ expression in naive T cells. 2) γδ+ T cells induce FoxP3+ Treg by IL-10 production. XCL1-produced by γδ T cells influence migration of tolerogenic XCR1+ DC to the mesenteric lymph node and consequently Treg induction. 3) Microbiota and production of IL-1β by macrophages in turn induce production of IL-2 by RORγt+ ILC3 followed by FoxP3+ Treg expansion. 4) IL-10-producing Breg promote class switching to suppress IgE production and maintain desensitization, while sustaining immunological tolerance. Breg are able to induce FoxP3+ Treg in an IL-10-dependent manner. 5) GATA3+ Treg reside in the small intestine and colon and have an important role in the maintenance of FoxP3 expression and Treg function by IL-2 production. 6) RORγt+ Treg are dependent on microbiota for maintenance and are sensitive to microbiota shifts. RA, TGF-β and IL-6 induce optimal differentiation of RORγt+ Treg. 7) GARP may induce both Foxp3+ Treg and LAP+ Treg. TGF-β and IL-10 may induce Foxp3+ Treg, LAP+ Treg and Tr1 cells. In addition, LAP+ Treg and Tr1 cells may transiently express the Foxp3 transcription factor. In inflammatory conditions, the presence of IL-6 and TGF-β may induce differentiation of naive T cells into Th17 phenotype, instead of FoxP3+ Treg. Moreover, Treg may lose Foxp3 expression under inflammatory conditions. Such “ex-FoxP3” cells may acquire a Th17 phenotype. IL-6 cytokine abolishes Foxp3+ Treg and LAP+ Treg induction by preventing GARP expression.
2.2. LAP+ Treg.
TGF-β is central to the induction and suppression mechanism of LAP+ Treg. Of the three different TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3), TGF-β1 is the most important in regulating immune responses [68]. TGF-β is first produced as pre-pro-TGF-β, which is transformed into pro-TGF-β dimers through homodimerization. Pro-protein convertase Furin cleaves the pro-TGF-β dimer into the carboxy-terminal dimer, or mature TGF-β; and amino-terminal dimer, LAP [88]. LAP remains non-covalently associated to mature TGF-β, thus preventing binding to the TGF-β receptor, forming a latent TGF-β complex (hence its name “latency-associated peptide”) [89]. Although surface LAP expression may be induced by TGF-β in a FoxP3-independent manner [90], both activated FoxP3+ Treg and FoxP3− Treg can express LAP [91, 92]. Furthermore, LAP+ cells are able to induce FoxP3 expression through a TGF-β-dependent mechanism [93]. The relationship between FoxP3+ Treg and LAP+ Treg remains incompletely understood. Both require TGF-β for induction and suppression and share the ability to produce IL-10, so it is plausible that there is plasticity between these cells (Figure 2). In addition, surface LAP expression has also been described in CD8+ T cells with suppressive properties [94].
LAP+ Treg were first reported to aid in the induction of peripheric tolerance in a murine model of experimental autoimmune encephalomyelitis (EAE) [95, 96]. Recently, these cells have been implicated in tolerance to oral antigen or anti-CD3 administration in animal models of EAE and autoimmune diabetes [1, 97]. The small intestine is the site for LAP+ Treg induction following oral antigen delivery [5] [34, 35, 37, 46, 98–100]. Administration of anti-LAP resulted in an increase in IL-17 and IFN-γ production, as well as abolishment of oral tolerance induction by anti-CD3 immunotherapy [101]. LAP+ Treg were found to be important in the suppression of inhibitory antibody formation against coagulation factor VIII and IX (FVIII and FIX) in oral tolerance for treatment of the inherited bleeding disorder hemophilia A and B in murine and canine pre-clinical models [5, 9, 34, 35, 37, 46], illustrating their utility in prevention of anti-drug antibody formation. Oral tolerance induction using FVIII domains expressed in tobacco chloroplasts revealed an increase in the frequency of circulating LAP-expressing Treg in tolerized hemophilia A mice, thus highlighting their potential use as an important cellular biomarker in human clinical trials for plant-based oral tolerance induction [35].
TGF-β activation involves release of mature TGF-β from LAP, either via DC or Treg in a cell intrinsic manner [102–104]. Receptor binding of mature TGF-β leads to phosphorylation of SMAD2 and SMAD3, which then form a trimeric structure with SMAD4. Subsequently, the SMAD complex translocates to the nucleus, where it may activate or repress gene expression [105]. TGF-β activation may also occur through GARP [106–108], which is expressed at a low level in resting Treg. It is known that GARP tethers LAP/TGF-β complex to the cell membrane, so that TGF-β activation might occur at the right time and the right place within close proximity of effector T cells, thereby allowing suppression in a TGF-β dependent manner [88, 109]. This mechanism would explain the need for cell-contact for induced Treg to exert their function.
As GARP is associated with FoxP3+ expression [109], most reports suggest that GARP is found on LAP+ Treg co-expressing FoxP3. However, Kuhn and collaborators developed an optimized in vitro model, in which CD4+ T cells expressing LAP do not co-express FoxP3. In this report, the in vitro induction of CD4+LAP+ T cells was abrogated by the IL-6 cytokine, which prevents GARP expression by STAT3-dependent inhibition of the Lrrc32 gene, which encodes the GARP protein. In vivo IL-6 and IL-6R deficiency induced an increase in CD4+LAP+ T cells, and in particular CD4+FoxP3+LAP+ T cells, thereby enhancing oral tolerance induction [110]. These reports show the key role of LAP+ Treg in oral tolerance induction, but they also raise questions about the plasticity and cooperation between LAP+ Treg and FoxP3+ Treg (Figure 2).
2.3. Tr1 cells
Type 1 regulatory T (Tr1) cells are a distinct subset of Treg that highly express IL-10 and that have been described in the context of mucosal antigen administration, including tolerance induction by nasal antigen or anti-CD3 administration [111, 112]. Tr1 cells are identified by surface co-expression of CD49b and lymphocyte activation gene 3 (LAG3) [113] (Figure 1), and may also express CTLA-4, programmed cell death protein 1 (PD-1), ICOS, early response gene 2 (Erg-2), and GATA-3 [114]. Tr1 induction or cell therapy can be used to prevent autoimmune disease or transplant rejection, and a number of clinical trials with antigen specific, allospecific, or polyclonal Tr1 cells have been assessed or are in clinical development (NCT02327221, NCT03198234, NCT01346085, NCT01656135) [114]. In plant cell-based oral tolerance, orally delivered antigen resulted in Tr1 (CD4+LAG-3+CD49+) cell expansion in LP, which locally upregulated IL-10 expression in a pre-clinical hemophilia B model [46]. However, its exact role in orally induced tolerance remains unclear.
Tr1 cell induction depends on IL-27 secreted by DCs, but not on FoxP3 expression [115, 116]. Although these cells may display transient expression of FoxP3 [117, 118], the transcription factor is not a prerequisite for the suppressive ability of Tr1 cells [119]. Tr1 cells induced by IL-27 and TGF-β produced by DC in lymph nodes or by IL-27 production by splenic macrophages has been observed in models of oral tolerance to food allergen [111, 120, 121]. IL-27 promotes Tr1 differentiation through induction of c-Maf, IL-21 and ICOS [122]. IL-27 also induces ligand-activated transcription factor aryl hydrocarbon receptor (AhR), which interacts with c-Maf and acts in synergy to induce Tr1 differentiation [123]. Tr1 cells induce immunosuppression mainly by producing the cytokine IL-10 and IL-21 [111, 123], which in turn inhibits IL-17 polarizing cytokines on DCs such as IL-1β, IL-6 and IL-23 (Figure 1) [124]. Besides high amounts of IL-10, Tr1 cells also secrete TGF-β upon TCR activation, thus exerting suppressive responses through release of both IL-10 and TGF-β [125].
3. Orally induced non-CD4 T cells with regulatory function
3.1. Regulatory CD8+ T cells
The majority of cells involved in oral tolerance are thought to be CD4+ T cells, but these may not be the only immune regulatory cells involved in oral immunotherapy. For example, it has been reported that CD8+ T cells with regulatory activity may be induced upon interaction with intestinal epithelial cells [126]. Regulatory CD8+ T cells express lower levels of FoxP3 compared to CD4+ Treg in mice, rats and humans [127]. In mice, surface markers such as CD122(+) or CD28(−) have been used to identify regulatory CD8+ T cells [128]. However, the complete definition of regulatory CD8+ T cells remains undefined [129]. Patients with IBD show defects in regulatory CD8+ T cells in the LP, which is associated with a breakdown of mucosal tolerance [130]. The regulatory role of CD8+ T cells was also shown in tolerance induction by oral administration of myelin basic protein (MBP) in experimental autoimmune encephalomyelitis [131]. Suppression of autoimmune encephalomyelitis was observed in recipient mice that received adoptive transfer of CD8+ cells from orally tolerized mice [131]. However, in vivo depletion of CD4+ T cells but not of CD8+ T cells completely abolished orally induced tolerance to ovalbumin (OVA) [42]. These reports imply that CD8+ Treg participate in but may not be essential to the development of oral tolerance.
3.2. γδ T cells
Gamma-delta TCR (γδ)-expressing T cells, representing only 1–2% of cells in secondary lymphoid tissues [132], have also been implicated in oral tolerance. γδ T cells display less TCR diversity, but can rapidly respond to pathogens at first contact, as well as self-molecules in response to danger signals or cellular stress through pattern recognition receptors [132, 133]. γδ T cells play an important role in gut homeostasis, as they are constitutively activated by normal microbiota of the intestinal lumen [134]. Interestingly, there is a subset of regulatory γδ T cells that expresses LAP (γδ+LAP+ cells) in the Peyer’s patches and small intestine. γδ+LAP+ cells themselves lack FoxP3 expression but can function as antigen presenting cells and are able to induce CD4+FoxP3+ T cells. γδ+LAP+-mediated induction of FoxP3+ Treg was shown to lead to amelioration of disease in an induced colitis model [135]. Further, γδ T cell deficiency [136] or depletion of γδ T cells [137] compromised orally induced tolerance to OVA in mice, whereas adoptive transfer of CD3+γδ+ spleen cells from tolerogenic mice into naive recipients restored tolerance [136]. The abolishment of antigen-induced oral tolerance in γδ T cell deficient mice was shown to be related to low levels of IL-10 production when low antigen-dose, but not high dose, was applied [137]. Moreover, recently published data demonstrated that anti-CD3 induced oral tolerance is also impaired in γδ T cell-deficient mice. γδ T cells enhance production of the chemoattractant XCL1/lymphotactin in the small intestine, which recruits tolerogenic XCR1+ DC to the mesenteric lymph node and consequently enhances Treg induction [138]. Importantly, the XCL1/XCR1 axis is required for orally induced tolerance by anti-CD3, but not by oral antigen administration [138] (Figure 2). Hence, these reports confirm that the mechanism of oral tolerance induction may vary somewhat depending on the approach.
3.3. Regulatory B cells
A possible regulatory role of B cells was already described in the 1970s [139], and more recently, B cells with immunosuppressive proprieties and intestinal immune tolerance have been termed regulatory B cell (Breg) or B10 cells, reflecting the production of IL-10 cytokine [140–142]. IL-10-producing Breg promote IgG4 isotype switching in human B cells, a non-inflammatory immunoglobulin isotype that suppresses IgE production [143]. For example, multiple studies on OIT based on sublingual immunotherapy (SLIT) have observed a significant increase in the level IgG4 in patients with food allergy [14, 144–147]. Although there was an initial increase of IgE levels, these subsequently declined, suggesting isotype switching. Discontinuation of therapy led to decrease of food-specific IgG4 levels in patients and loss of tolerance, suggesting that IgG4 plays a role in maintaining desensitization and sustaining immunological tolerance [148]. Furthermore, it is known that the production of antigen-specific IgA and secretory IgA (S-IgA) in the intestines prevents uptake of antigen across the epithelium, thereby preventing inflammatory responses [149]. Indeed, significant increases in allergen-specific IgA have been reported in SLIT [150, 151]. Oral administration of casein for treatment of cow’s milk allergy resulted in increase of IL-10 producing CD5+ B cells in MLN in casein tolerogenic mice. The adoptive transfer of mesenteric CD5+ B cells from casein-tolerized mice suppressed allergic responses via induction of FoxP3+ Treg in an IL-10 dependent manner [152]. Other reports also suggest that Breg can induce FoxP3+ Treg expansion [153, 154] (Figure 2).
3.4. Innate lymphoid cells
Innate lymphoid cells (ILC) have been linked to maintenance of the integrity of intestinal epithelial barriers through commensal microbiota signals, and promoting adaptive immunity [155]. In response to colonization of the gut by Clostridia species, RORγt-expressing ILC (RORγt+ ILC3) and T cells upregulate IL-22 production in the intestinal LP, thereby reducing sensitization to oral antigens [156]. RORγt+ ILC3 have been associated with the success of oral tolerance to dietary antigen. In the small intestine environment, macrophages produce IL-1β in response to microbiota signals. IL-1β activates IL-2-producing RORγt+ ILC3, which in turn triggers FoxP3+ Treg expansion in the small intestine [157]. Furthermore, IL-1β induces GM-CSF production in ILC3 cells, which is a crucial cytokine for CD103+ DC differentiation [157] (Figure 3). Thus, failure to produce IL-1β by macrophages results in reduced production of GM-CSF and IL-2 by RORγt+ ILC3, which in turn impedes FoxP3+ Treg expansion and impaired ability to induce oral tolerance. Similarly, genetic ablation of il-2 or csf2 in ILC3 cells decreases accumulation of intestinal Treg upon oral administration of antigen [157]. Thus, RORγt+ ILC3-derived IL-2 plays a role in maintaining Treg homeostasis and promoting oral tolerance in the small intestine. Therefore, crosstalk between innate myeloid and lymphoid cells is an interesting field to be explored in OIT (Figure 2).
Figure 3. Schematic overview of oral tolerance development/oral Treg induction.
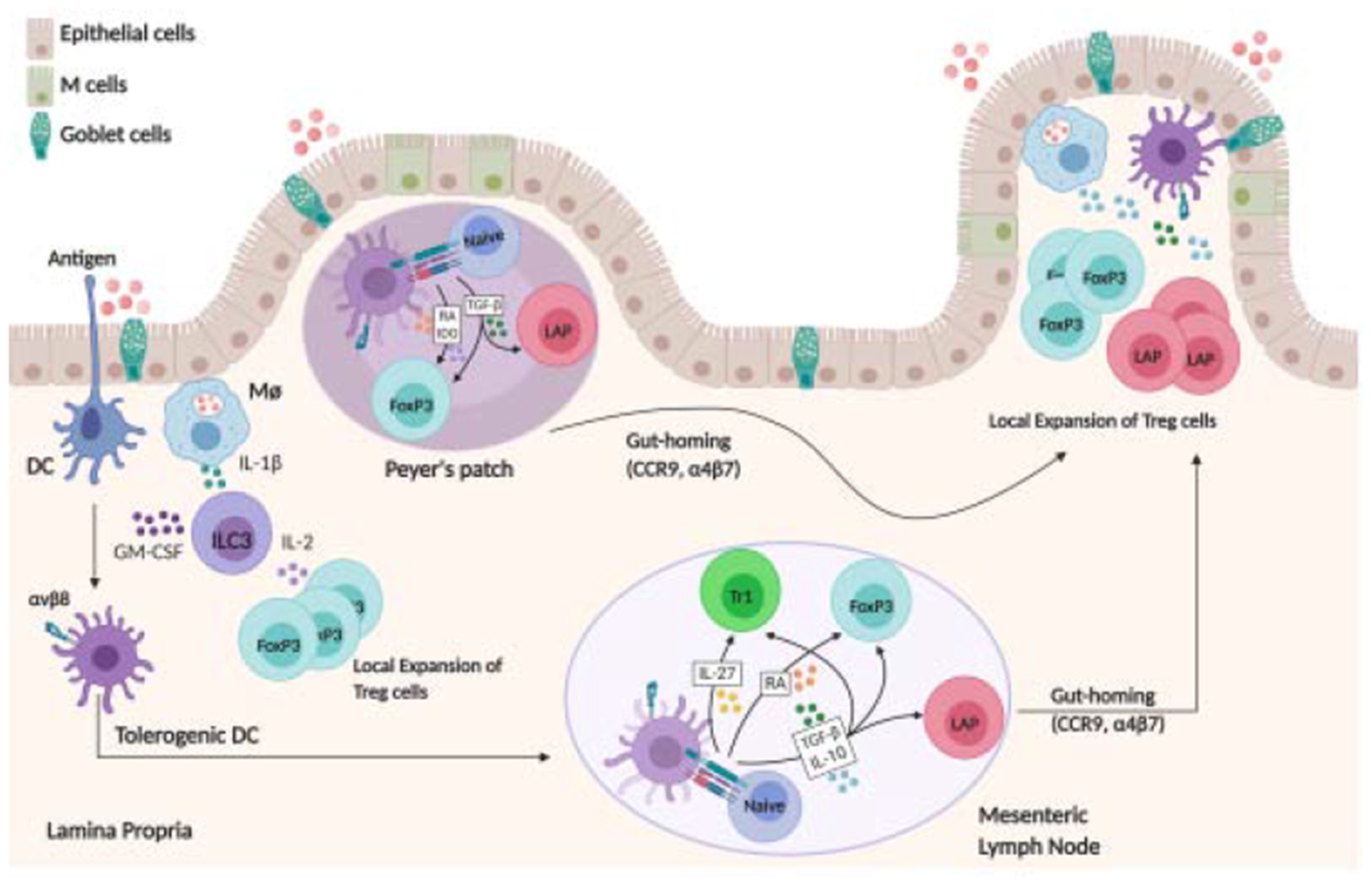
Upon ingestion and the digestive process, antigen reaches the small intestine and can be taken up directly by phagocytes or pass through goblet cell associated passages prior to capture by DC in LP. Microbiota induced IL-1β-secreting intestinal macrophages can induce CD103+ DC differentiation by stimulating GM-CSF-producing ILC3. Moreover, IL-1β-secreting intestinal macrophages induce IL-2 production by ILC3, which in turn stimulates FoxP3+ Treg expansion. Antigen is presented to T cells in the Peyer’s patches or in the MLN through tolerogenic CD103+ DCs, which express the integrin αvβ8 able to activate TGF-β from the latent form and induce Treg. CD103+ DCs produce IDO and RA, which in combination with TGF-β induces FoxP3 Treg differentiation. TGF-β by itself can induce LAP+ Treg differentiation. Tr1 differentiation is triggered by IL-27 producing tolerogenic DCs. CD103+ DCs also mediate upregulation of gut-homing receptors, CCR9 and α4β7 integrin, allowing the recently primed Tregs to home back to the LP, where they undergo local expansion to induce oral tolerance.
4. Other regulatory cells that likely assist in orally induced tolerance
4.1. RORγt-expressing Treg
The abundant microbial community found on intestine plays a prominent role in the regulation of oral tolerance. Retinoic acid-related orphan receptor gamma t (RORγt)-expressing Treg (RORγt+ Treg) represent a substantial number among Treg in the intestine and are dependent on the microbiota for maintenance and thus might be sensitive to microbiota shifts [3, 53, 158, 159]. Clostridia and Helicobacter species are examples of bacteria that may induce RORγt+ Treg differentiation in the gut [156, 160–162], and use of broad-spectrum antibiotics may potentially deplete RORγt+ Treg [158]. RORγt is a transcriptional factor expressed in both Treg and Th17 cells. CD103+ DC are able to generate RA through enzymatic activity of retinal dehydrogenases (RALDH) and, in synergy with TGF-β and IL-6, induces optimal differentiation of RORγt+ Treg [50]. Furthermore, RORγt+ Treg are associated with IL-10 production [163], which may assist FoxP3+ Treg differentiation (Figure 2). While it has become evident that RORγt+ Treg play a crucial role in induction and maintenance of tolerance and homeostasis in the intestine with regard to responses to bacteria, the role of these cells in orally induced tolerance is unknown.
4.2. GATA3+ Treg
Although representing only a minor fraction of the overall Treg population, GATA3+ Treg reside in the small intestine and colon and are involved in control of chronic inflammation [50]. Additionally, GATA3 has an important role in maintenance of FoxP3 expression and Treg function at sites of inflammation [164, 165]. GATA3+ Treg require IL-2 for persistence, hence IL-2-deficient mice show a robust decrease of this subset in the small intestine [165]. Conversely, in vivo systemic administration of IL-2 induces an increase of Treg-expressing GATA in the spleen [165]. In orally induced tolerance, in vivo administration of anti-IL-2 resulted in abolishment of conversion of antigen-specific FoxP3− to FoxP3+ CD4+ T cells [166]. It is therefore plausible that GATA3+ Treg contribute to the generation of FoxP3 expressing cells in the intestine during oral tolerance induction (Figure 2).
5. Mechanisms of oral Treg induction
Orally induced Treg cells have a crucial role in maintaining tolerance to microbiota, diet and other harmless antigens [50]. Here, we describe mechanisms that mediate Treg differentiation during OIT.
5.1. Immune cells promoting Treg induction and expansion
CD103+ DCs are considered major drivers of tolerance in the intestine. Intestinal DC express the integrin αvβ8, which has a crucial role in activation of TGF-β from the latent form and in the generation of Treg [102, 167, 168]. Both DCs and macrophages in the LP express aldehyde dehydrogenase (ALDH), which mediates RA production from dietary vitamin A [60, 62]. RA production by CD103+ DCs in combination with TGF-β induces FoxP3+ expression in naive T cells and promotes tolerogenic environment in the intestine [60–62]. RA-produced by CD103+ DCs also mediates pTreg migration in the LP through upregulation of gut-homing receptors, CCR9 and α4β7 integrin [61, 63]. In fact, ablation of CCR9 or β7 integrin impairs oral tolerance development [76, 169]. CD103+ DCs also mediates differentiation of LAP+ Treg in gut draining lymph nodes, and their activation promotes expression of gut homing receptors [13, 46]. Finally, CD103+ DCs express high levels of the enzyme indoleamine 2,3-dioxygenase (IDO), which metabolizes tryptophan. IDO activity contributes to differentiation of FoxP3+ Treg and the suppression of Th1 and Th17 cells [170, 171] (Figure 3).
Oral tolerance involves antigen uptake by oral administration, capture by intestinal DC and presentation to T cells in the Peyer’s patches or in MLN. However, free antigen can also enter the blood vessels in the LP and reach the liver, where it is captured by plasmacytoid DC (pDC) [172]. pDC are important mediators of innate immune responses against pathogens but also play an important role in immune regulation [173]. [173]. Although pDC play a major role in TLR7 and 9 mediated sensing of viral RNA and DNA, leading to a robust type I interferon (IFN-I) response in the periphery, studies reveal that pDC mediate an immunoregulatory profile in the gut, thus suppressing T cell responses [174, 175]. Factors produced in mucosal sites, such as IL-10 and TGF-β inhibit IFN-I production, but do not block the ability of pDC to induce Treg differentiation [174, 176]. Thus, pDCs may contribute to oral tolerance development through enhancing the induction of Treg differentiation [172, 177, 178]. pDC express TGF-β and ALDH enzyme, which have a key function in Treg induction as mentioned above. Studies by Uto and colleagues demonstrated that pDC induce oral tolerance through expansion of FoxP3+ Treg in a food allergy model [179] and their frequencies are increased in plant-based oral tolerance [46]. Similarly, oral administration of Lactobacillus gasseri enhances accumulation of pDCs and Tr1 cells in the intestinal LP in a food allergy model [180].
The intestine is also populated with other subsets of DC, including XCR1+ and CD103− DC, which also express enzymes required for RA formation [181]. Further studies are required to better understand the role of these cells in OIT.
In addition to the critical role of DCs in oral Treg induction, there is evidence that regulatory lymphocytes impact each other’s induction. FoxP3+ Treg generated outside the intestinal immune system are necessary for the generation of intestinal Treg. For example, Edwards and colleagues demonstrated that ablation of Treg or specific deletion of TGF-β in FoxP3+ Treg decreased de novo generation of FoxP3+ Treg in the GALT of OVA-fed mice and impaired oral tolerance induction [166]. Induction of antigen specific Treg from CD4+FoxP3− effector T cells occurs by conversion in the presence of antigen and FoxP3+ Treg. This process of infectious tolerance is thought to require interaction of FoxP3+ Treg and the effector T cell with a common antigen presenting cell (APC) [182]. Enhanced consumption of essential amino acids by Treg and APC has also been associated with induced Treg generation and inhibition of T cell proliferation [183]. The role of infectious tolerance in Treg cells induction during OIT remains to be further elucidated.
5.2. Diet-derived mediators
Early contact with dietary antigens induces pTreg development and accumulation in the intestinal LP [53]. However, continuous generation of pTreg requires constant contact with dietary antigens, which preserve the accumulation of these cells in the LP [3, 53, 158]. Furthermore, depletion of dietary antigens increases susceptibility to intestinal allergy by inducing enhanced accumulation of Th1 cells and decreased number of pTreg in the intestine in an experimental model of food allergy [53]. Thus, the immune response to newly introduced dietary antigens is suppressed by pTreg in the LP, which are generated with a continuous exposure to antigens derived from diet.
Diet-derived metabolites, including vitamin A, D3 and B3 influence various immune functions that include Treg. Vitamin A (retinol) is taken up through diet and converted to RA in a two-step enzymatic reaction. Retinol is metabolized to retinal by alcohol dehydrogenase (ADH), which is ubiquitously expressed, and then the retinal dehydrogenases (RALDH) catabolize the final conversion of retinal to RA [184, 185]. RA plays a crucial role in maintenance of mucosal tolerance in the intestine through induction of FoxP3+ Treg [53, 185]. Specifically, RA mediates Treg differentiation through binding to the nuclear RA receptors (RAR) and the retinoid X receptor (RXR), which promotes FoxP3 expression [63, 64, 79]. In vivo administration of RA promotes expansion and intestinal homing of FoxP3+ Treg in OVA immunized mice [63]. Furthermore, vitamin A-deficient diets decrease expansion of FoxP3+ Treg and LAP+ Treg and promote Th17-mediated inflammation, while vitamin A supplementation enhances accumulation of Treg in the GALT [3, 186].
Niacin or vitamin B3, which is known to have anti-inflammatory properties [187], binds to GPR109A on DCs in the LP. This interaction promotes RALDH expression, which mediates formation of RA and enables DCs to induce FoxP3+ Treg and IL-10 production in T cells [188]. Genetic ablation of GPR109A leads to impaired oral tolerance and enhanced susceptibility to colitis in a murine model [188]. The receptor of vitamin B9 or folic acid, which is derived from diet and bacterial commensal metabolites, is also highly expressed on Treg and its known to enhance Treg survival through upregulation of the anti-apoptotic BCL-2 [189]. Mice fed with folic acid-deficient diet have decreased accumulation of FoxP3+ Treg expressing IL-10 in the colon, which is associated with an enhanced susceptibility to intestinal inflammation. Furthermore, folic acid supplementation enhances Treg accumulation in the colon by inhibiting apoptosis [189].
5.3. Microbiota
The GI compartment is colonized by a large number of commensal microbiota, which are a large source of foreign antigens and are known to promote oral tolerance [61, 62, 160, 188]. Commensal microbiota produce several factors, such as polysaccharide A, short chain fatty acids and RA, which regulate innate and adaptive immune cells, and their dysbiosis has been associated with an increased incidence of food allergy [190]. For example, Zhou and colleagues demonstrated that gut-microbiota induce IL-1β production by intestinal macrophages. IL-1β activates IL-2 producing ILC3, which in turn triggers FoxP3+ Treg expansion in the small intestine [157]. Furthermore, IL-1β induces GM-CSF production in ILC3 cells, which is a crucial cytokine for CD103+ DC differentiation. Thus, the initial crosstalk between myeloid cells and ILC3 plays a key role in the expansion of Treg cells and the induction of oral tolerance induced by dietary antigens. However, further studies are necessary to identify additional interactions between these cells. Colonization by certain species of commensal bacteria such as Clostridia and Bacteroides enhances accumulation of intestinal Treg, thereby promoting immune homeostasis in the intestine [5, 158, 190, 191]. Treg numbers are decreased in the small intestine of germ-free mice compared to specific pathogen-free (SPF) mice [160, 192]. However, restoration of microbiota induces an enhanced expansion of Treg and restores immune homeostasis in germ-free mice [160]. Song and colleagues found that intestinal microbiota mediated formation of gut bile-acid metabolites from the diet, which are essential for Treg expansion in the intestine [192]. Thus, immune cells activated by microbiota triggers Treg differentiation and its function in the intestine during oral tolerance induced by dietary antigens.
Bacteria can also influence the suppressive phenotype of Treg. For instance, colonization of Bacteroides fragilis, a human symbiont, promotes accumulation of IL-10-producing FoxP3+ Treg in the intestine [70]. Other species of commensal microbiota, like Clostridia also facilitate differentiation of Treg and expression of immunosuppressive molecules ICOS, CTLA4, LAG-3, PD-1, IL-10 and TGF-β [49, 70, 193]. Expression of these surface molecules inhibits T cell activation and promotes maintenance of intestinal homeostasis [68]. Thus, there is a mutual relationship between commensal bacteria and intestinal Treg, which together promotes intestinal homeostasis. However, the exact mechanisms by which microbiota-derived signals induce functional Treg still need to be determined. Commensal bacteria also play a key role in Treg induction to plant cell-derived antigens. Plant cells provide natural bioencapsulation through their cell wall, which protects antigens from degradation by stomach acids. However, once plant cells reach the small intestine, bacterial enzymes are required to at least partially degrade cell wall components so that antigens are released and can be delivered to the immune system. While such enzymatic activity is most abundant in the colon, there is recent evidence for presence of several bacterial orders in the small intestine, in particular in the duodenum, including producers of cellulase, β-N-acetylhexosaminidase, amino-acid N-acetyltransferase, β-glucosidase, xylan 1,4-β-xylosidase, and pectinesterase [5]. These are distinct from bacteria that predominantly produce these enzymes in the colon. In addition, plant cells are a source of vitamins that can affect immune function such as provitamin A carotenoids, a precursor of retinol/retinoic acid.
6. Mechanisms of suppression
Treg preserve homeostasis in the intestinal tract through multiple suppressive mechanisms: production of inhibitory cytokines IL-10, IL-35 and TGF-β; apoptosis or anergy of effector T cells, perforin and granzyme-dependent cytolysis of target cells; suppression of DC maturation and function through expression of PD-1, CTLA4, LAG3; and apoptosis of effector T cells by deprivation of IL-2. Of these, inhibitory cytokines have been studied the most in intestinal Treg.
6.1. Cytokines mediating Treg suppressor activity
IL-10 is an anti-inflammatory cytokine produced by many cells, including FoxP3+ Treg, LAP+ Treg and Tr1 cells. IL-10 exerts its function by inhibiting Th1 and Th17 responses and downregulating MHC-II expression in monocytes [194–196]. Expression of IL-10 in Treg is regulated by STAT3 [194] and is crucial for the maintenance of intestinal homeostasis. Studies in humans and mice have shown that mutations of IL-10R leads to development of severe colitis and enhances accumulation of Th17 in intestine [193, 195, 197]. Furthermore, absence of IL-10R or STAT3 in APCs in the LP exacerbates intestinal inflammation [198, 199]. Th17 cells also express IL-10R, and mutations in this receptor enhance Th17 cell proliferation in the intestine, so that IL-10 produced by Treg plays a key role in suppression of Th17. Indeed, IL-10RA or STAT3 deficiency in Treg induced increased activation and accumulation of Th17 cells in the intestine in an animal model of colitis [194, 195]. The relevance of IL-10 for the function of orally induced Treg is evident from the observation that OIT with anti-CD3 leads to induction of IL-10 producing LAP+ Treg, which prevents T cell activation and protects against inflammatory conditions [98, 99]
TGF-β has been broadly described as an immunomodulator of effector T cell activation with key roles in OIT [200]. TGF-β is responsible for FoxP3+ and LAP+ Treg induction and differentiation and most Treg types are able to produce this cytokine (Figure 1). Oral administration of anti-CD3 enhances regulatory functions by induction of TGF-β produced by LAP+ Treg, which contributes to protection against inflammation in murine models of EAE and colitis [98, 99]. Depletion of LAP+ Treg or TGF-β enhances T cell proliferation and exacerbates inflammatory diseases [98, 99, 200].
IL-35 is an immunoregulatory cytokine secreted specifically by FoxP3+ Treg, and its role in Treg suppressive activity has been broadly studied [201, 202]. The importance of IL-35 for Treg was initially documented by Collison and colleagues (2007), who demonstrated that deletion of Ebi3, the IL-27B subunit of IL-35, reduced Treg activity in vitro and impaired intestinal homeostasis in vivo [201]. More recently, Wei et al (2017) found that expression of IL-35 depends on FoxP3, while IL-10 production by Treg depends on the c-Maf and Blimp-1 transcription factors [191, 202]. Furthermore, deletion of IL-35 producing Treg or genetic deletion of Blimp-1 in Treg enhances production of IL-17 and IFN-γ and worsens colitis [202].
6.2. Induction of anergy and effector T cell apoptosis
Antigen dose is an important factor for suppressive mechanisms. Low antigen dose favors active suppression with Treg induction whereas a high dose favors anergy or deletion of antigen-specific T cells [203, 204]. In oral tolerance induction using low antigen dose, the suppressive effect was associated with IL-10 secreting cells in Peyer’s patches [205]. In EAE, TGF-β-secreting regulatory cells were found in Peyer’s patches after low doses of orally administered MBP, whereas this was not observed after high dose oral administration [206]. Benson and colleagues also demonstrated that oral administration of MBP reduces antigen-specific TCR expression in CD4+ T cells in the peripheral LNs of a mouse model of EAE [207]. In human food allergy studies, high dose oral desensitization along with omalizumab (anti-IgE monoclonal antibody) reduced Th2 responses, which was associated with anergy/depletion of allergen-specific T cells [208]. Patients with peanut-allergy achieved successfully OIT by inducing an anergic Th2 cell phenotype, while no induction of antigen-specific Treg was observed [209].
6.3. Bystander suppression
Bystander suppression is a mechanism in which antigen specific Treg generate a nonspecific antigen effect [210]. This suppression mechanism requires colocalization of tolerogen and the unrelated antigen [211, 212]. Hemagglutinin immunized mice showed decreased specific T cell proliferation when mice were also given OVA OIT [210]. More recent studies demonstrated that antigen-specific Treg cells induced during OVA administration are recruited into the airway mucosa and control lung inflammation in an airway allergy mouse model [212]. This mechanism of suppression is also associated with an enhanced production of IL-10 and TGF-β, which aids in inhibition of the unrelated T cell activation [210].
6.4. Other suppressive mechanisms
In order to preserve immune homeostasis in the intestine, Treg modulate microbiota diversity by induction of IgA production. For example, Kawamoto et al. (2014) demonstrated that transfer of Treg, but not T naive cells, enhanced the diversity of bacterial species in Cd3e deficient mice. Furthermore, transferred FoxP3+ Treg increased germinal center (GC) formation and induced IgA production in the small intestine [213]. Several studies have shown the role of granzyme in controlling effector T cells [214, 215]. Treg can regulate inflammation by inducing cytolysis of effector T cells through production of granzyme A and B [215]. However, the role of granzyme-dependent cytolysis of target cells in the maintenance of oral tolerance remains unknown.
7. Examples of OIT-induced Treg in treatment of disease
OIT can be used to alleviate inflammatory disease in an antigen-nonspecific fashion by non-specific enhancement of Treg [1, 97, 216]. This can be accomplished by supply of IL-2, which is critical for Treg development, expansion and maintenance of suppressive function [49, 217, 218]. Several studies have shown that low dose subcutaneous injections of IL-2 leads to an increase in number and function of Treg, thereby ameliorating autoimmune disease in patients [12, 15]. Bonnet and colleagues found that low dose IL-2 enhanced accumulation of FoxP3+ Treg in the GALT and prevented food allergy in two different experimental animal models of allergy [216]. An alternative approach is oral administration of anti-CD3 [98, 99]. The mechanism by which systemic anti-CD3 mediates tolerance is not well understood but is thought to include a combination of apoptosis and anergy of T cells. While the use of anti-CD3 for Treg induction may appear paradoxical, given its role in depleting T cells, it is thought that CD4+FoxP3+ Treg express CD3 at lower levels as compared to CD4+FoxP3− effector T cells, thus rendering them less susceptible to anti-CD3 mediated apoptosis [219]. The tolerogenic microenvironment caused by anti-CD3 treated apoptotic T cells and phagocytic macrophages is also thought to lead to TGF-β production inducing FoxP3 in CD4+ T cells, thus rendering them suppressive. TGF-β can further skew DC into a tolerogenic phenotype [220]. Humanized anti-CD3 treatments like otelixizumab, teplizumab and visilizumab are being evaluated for transplant rejection and autoimmunity (NCT01287195, NCT00129259, NCT04270942). In contrast to systemic delivery, oral anti-CD3 treatment does not cause depletion of T cells [98], although it does increase the incidence of LAP+ Treg in the GALT [101], which limits inflammation by regulating T cell activation via TGF-β and IL-10 production [98, 99]. Nasal administration of anti-CD3 or antigen also enhances expansion of different types of Treg, including LAP+ Treg and Tr1 cells [11, 100]. Illustrating the importance of TGF-β in the anti-CD3 approach, blockage of TGF-β abolished oral tolerance induction and increased inflammation in autoimmune disease [11, 100].
Antigen-specific treatment by oral administration in OIT has been broadly explored as a means of tolerance induction, as it can elevate numbers and improve function of antigen-specific Treg, such as increase of hypomethylation of FoxP3 gene and thus enhanced expression [221, 222]. OIT seeks to expand pTreg in order to alleviate various autoimmune and allergic conditions, as well as prevent anti-drug antibody formation. For example, peanut OIT mitigates food allergies in part through induction of antigen specific FoxP3+ Treg and reduction of basophil activation in patients with peanut allergy [221, 222]. Recently the Food and Drug Administration (FDA) approved an oral immunotherapy termed (AR101, Palforzia) for the reduction of allergic reaction incidence in patients with peanut allergy [223]. Peanut OIT increases the migration of Treg toward the intestinal epithelium and enhances the suppressive capacity of Treg in allergic patients submitted to peanut OIT.
Another application of antigen-specific oral administration is the prevention of anti-drug antibodies known as “inhibitors” that form in a proportion of hemophilic patients as a result of intravenous coagulation factor replacement therapy [34, 35, 224, 225]. In order to orally induce Treg while avoiding expensive production of recombinant proteins and protecting antigen from degradation in the stomach prior to reaching the small intestine, a plant cell-based approach was developed [34, 226]. Chloroplasts of transgenic tobacco or lettuce plants expressing high levels of the mature sequence of human FIX for tolerance induction in hemophilia B or the heavy chain and C2 domain of the human FVIII for hemophilia A were expressed as N-terminal fusions to the cholera non-toxic B subunit (CTB) to target the GM1 receptor of intestinal epithelial cells for enhanced transmucosal delivery. The plant cell wall provides natural bioencapsulation that can resist breakdown by stomach acids. However, enzymatic activities provided by commensal bacteria release antigen into the small intestine, where up-take and translocation to the immune system was observed in LP and Peyer’s patches [5, 46]. As a result, antigen was delivered to intestinal DCs, including CD103+ DCs, and increased frequencies of CD103+ DCs and pDCs were observed in mesenteric lymph nodes of hemophilic mice upon challenge with intravenous antigen [5, 34, 35, 37, 46]. Activated CD103+ DC migrated to draining lymph nodes and induced Treg expansion and migration. Uptake of orally administered coagulation factor induced IL-10 and TGF-β producing LAP+ T cells, FoxP3+ Treg, and T cells expressing gut-homing receptors [5, 34, 35, 37, 46]. Adoptive transfer studies demonstrated that suppression of antibody formation was achieved by induced FoxP3+ and LAP+ Treg, with LAP+ Treg providing most of the suppression [5, 46]. Ultimately, this resulted in suppression of inhibitor formation against FVIII and FIX in murine and canine models of hemophilia, and also in suppression of IgE formation and anaphylactic reactions against FIX [34, 36, 37].
However, there are some limitations in OIT including dosing frequency and lack of long-term tolerance. In plant cell-based therapy, prolonged oral delivery is required for long-lasting tolerance as an increase in inhibitor formation was observed once oral delivery was discontinued [34, 46]. As the use of adjuvant allows lower and shorter dosing regimens, further approaches could consider the use of adjuvant as an alternative to overcome the long-term tolerance issue.
8. Conclusions and future perspectives
In the past decade, there has been an increase in knowledge regarding OIT, as well as mechanisms that mediate the development of this process. Three distinct Treg subsets have been shown to majorly contribute to OIT: FoxP3+ Treg, LAP+ Treg and Tr1 cells. These cells share several key characteristics and functions, such as the ability to produce IL-10 and TGF-β, which are critical mediators of immune suppression in OIT. TGF-β is also a key cytokine for FoxP3+ and LAP+ Treg differentiation. CD103+ DC and pDC have been shown to be key drivers of Treg differentiation and expansion in the intestine. Crosstalk between these cells and ILC, as well as between Treg subsets in the intestinal compartment is integral for the success of OIT. Future research may therefore focus on further understanding of this cellular crosstalk in order to improve the OIT approach and achieve lasting tolerance. Although the underlying mechanisms of OIT have not been fully elucidated, it is recognized as a promising strategy, which has resulted in several clinical trials including peanut allergy (FDA approved AR101), type 1 diabetes (NCT00223613, ISRCTN76104595 NCT02547519, NCT03364868), and arthritis (NCT00000435), demonstrating the importance of OIT in the improvement of allergic and inflammatory conditions [227, 228]. Other routes of antigen administration known to induce tolerance have been used for ongoing clinical trials in T1D and in multiple sclerosis, such as intradermal (NCT01536431, NCT01973491) and subcutaneous (NCT00435981, NCT0072341, NCT00529399) administration of the antigen. These routes of antigen administration have been successful in induce FoxP3+ Treg and enhance IL-10 production. However, more studies are required to address the mechanisms of tolerance induced by these pathways and the differences with OIT. In summary, critical questions remain not only regarding mechanisms, but also optimal antigen dose selection, route of administration and responsiveness to the treatment. These will aid in identifying new targets for treatment and development of new treatment protocols that are safer and more effective.
Highlights:
Oral antigen-based oral immunotherapies (OITs) alleviate allergies and autoimmune diseases.
OIT induces expansion of several subtypes of regulatory T cells (Treg) such as FoxP3+ and LAP+ Treg and Tr1 cells.
Orally induced Treg exhibit overlapping function and cooperation between each other.
Tolerogenic dendritic cells are drivers of Treg cells expansion and differentiation in the small intestine.
TGF-β, RA, IL-27 and IDO determine differentiation of orally induced Treg.
Role of intestinal microbiota in oral Treg induction remains to be defined.
Acknowledgments
This work was supported by NIH grants R01 HL133191 and R01 HL107904 to HD and RWH; R01 HL131093 to RWH and CT; and U54 HL142012 to RWH. Figures were generated using BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiner HL, et al. , Oral tolerance. Immunol Rev, 2011. 241(1): p. 241–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noval Rivas M, et al. , Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity, 2015. 42(3): p. 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnmacht C, et al. , The microbiota regulates type 2 immunity through RORγt+ T cells. Science, 2015. 349(6251): p. 989–993. [DOI] [PubMed] [Google Scholar]
- 4.Chang SY, et al. , Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity, 2013. 38(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar SRP, et al. , Role of Small Intestine and Gut Microbiome in Plant-Based Oral Tolerance for Hemophilia. Front Immunol, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keeffe MS, et al. , SLAMF4 Is a Negative Regulator of Expansion of Cytotoxic Intraepithelial CD8+ T Cells That Maintains Homeostasis in the Small Intestine. Gastroenterology, 2015. 148(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn C and Weiner HL, Immunology. How does the immune system tolerate food? Science (New York, N.Y.), 2016. 351(6275). [DOI] [PubMed] [Google Scholar]
- 8.Wang X, et al. , Mechanism of oral tolerance induction to therapeutic proteins. Advanced drug delivery reviews, 2013. 65(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniell H, Kulis M, and Herzog RW, Plant cell-made protein antigens for induction of Oral tolerance. Biotechnol Adv, 2019. 37(7): p. 107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pabst O and Mowat AM, Oral tolerance to food protein. Mucosal Immunology, 2012. 5(3): p. 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong Y, et al. , CD4+LAP + and CD4 +CD25 +Foxp3 + regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE−/− mice. Journal of Clinical Immunology, 2012. 32(5): p. 1104–1117. [DOI] [PubMed] [Google Scholar]
- 12.Castela E, et al. , Effects of Low-Dose Recombinant Interleukin 2 to Promote T-Regulatory Cells in Alopecia Areata. JAMA Dermatology, 2014. 150(7): p. 748–751. [DOI] [PubMed] [Google Scholar]
- 13.Tordesillas L, et al. , Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. Journal of Allergy and Clinical Immunology, 2017. 139(1): p. 189–201.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EH, et al. , Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol, 2011. 127(3): p. 640–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humrich JY, et al. , Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Annals of the Rheumatic Diseases, 2015. 74(4): p. 791–792. [DOI] [PubMed] [Google Scholar]
- 16.Wells HG and Osborne TB, The Biological Reactions of the Vegetable Proteins I. Anaphylaxis. 1911, The Journal of Infectious Diseases, Volume 8, Issue 1, 3 January 1911, Pages 66–124. [Google Scholar]
- 17.CHASE MW, Inhibition of experimental drug allergy by prior feeding of the sensitizing agent. Proc Soc Exp Biol Med, 1946. 61: p. 257–9. [DOI] [PubMed] [Google Scholar]
- 18.David MF, Prevention of homocytotropic antibody formation and anaphylactic sensitization by prefeeding antigen. J Allergy Clin Immunol, 1977. 60(3): p. 180–7. [DOI] [PubMed] [Google Scholar]
- 19.Thomas HC and Parrott MV, The induction of tolerance to a soluble protein antigen by oral administration. Immunology, 1974. 27(4): p. 631–9. [PMC free article] [PubMed] [Google Scholar]
- 20.André C, et al. , A mechanism for the induction of immunological tolerance by antigen feeding: antigen-antibody complexes. J Exp Med, 1975. 142(6): p. 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husby S, et al. , Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol, 1994. 152(9): p. 4663–70. [PubMed] [Google Scholar]
- 22.Vickery BP, et al. , Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol, 2017. 139(1): p. 173–181 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bird JA, et al. , Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J Allergy Clin Immunol Pract, 2018. 6(2): p. 476–485 e3. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, et al. , Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol J, 2011. 9(9): p. 982–90. [DOI] [PubMed] [Google Scholar]
- 25.Lee CC, et al. , Construction of a Der p2-transgenic plant for the alleviation of airway inflammation. Cell Mol Immunol, 2011. 8(5): p. 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakasa Y, et al. , Oral immunotherapy with transgenic rice seed containing destructed Japanese cedar pollen allergens, Cry j 1 and Cry j 2, against Japanese cedar pollinosis. Plant Biotechnol J, 2013. 11(1): p. 66–76. [DOI] [PubMed] [Google Scholar]
- 27.Halota W, et al. , Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial. J Viral Hepat, 2015. 22(8): p. 651–7. [DOI] [PubMed] [Google Scholar]
- 28.Lalazar G, et al. , Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol, 2015. 35(4): p. 399–407. [DOI] [PubMed] [Google Scholar]
- 29.Su J, et al. , Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol J, 2015. 13(8): p. 1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iizuka M, et al. , Suppression of collagen-induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of type II collagen. Plant Biotechnol J, 2014. 12(8): p. 1143–52. [DOI] [PubMed] [Google Scholar]
- 31.Hansson C, et al. , Feeding transgenic plants that express a tolerogenic fusion protein effectively protects against arthritis. Plant Biotechnol J, 2016. 14(4): p. 1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. , Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice. Diabetologia, 2018. 61(6): p. 1384–1396. [DOI] [PubMed] [Google Scholar]
- 33.Posgai AL, et al. , Plant-based vaccines for oral delivery of type 1 diabetes-related autoantigens: Evaluating oral tolerance mechanisms and disease prevention in NOD mice. Sci Rep, 2017. 7: p. 42372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman A, et al. , Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood, 2014. 124(10): p. 1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon KC, et al. , Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol J, 2018. 16(6): p. 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog RW, et al. , Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce. Mol Ther, 2017. 25(2): p. 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su J, et al. , Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials, 2015. 70: p. 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satitsuksanoa P, et al. , Regulatory Immune Mechanisms in Tolerance to Food Allergy. Front Immunol, 2018. 9: p. 2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni DH, et al. , Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal immunology, 2020. 13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macpherson AJ and Uhr T, Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science, 2004. 303(5664): p. 1662–5. [DOI] [PubMed] [Google Scholar]
- 41.Schiavi E, Smolinska S, and O’Mahony L, Intestinal dendritic cells. Curr Opin Gastroenterol, 2015. 31(2): p. 98–103. [DOI] [PubMed] [Google Scholar]
- 42.Garside P, et al. , CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol, 1995. 7(3): p. 501–4. [DOI] [PubMed] [Google Scholar]
- 43.Curotto de Lafaille MA, et al. , Adaptive Foxp3+ regulatory T cell-dependent and - independent control of allergic inflammation. Immunity, 2008. 29(1): p. 114–26. [DOI] [PubMed] [Google Scholar]
- 44.Fukaura H, et al. , Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest, 1996. 98(1): p. 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiner HL, Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect, 2001. 3(11): p. 947–54. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. , Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood, 2015. 125(15): p. 2418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, et al. , Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med, 2003. 198(12): p. 1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oida T, et al. , CD4+CD25−T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol, 2003. 170(5): p. 2516–22. [DOI] [PubMed] [Google Scholar]
- 49.Tanoue T, Atarashi K, and Honda K, Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol, 2016. 16(5): p. 295–309. [DOI] [PubMed] [Google Scholar]
- 50.Whibley N, Tucci A, and Powrie F, Regulatory T cell adaptation in the intestine and skin. Nat Immunol, 2019. 20(4): p. 386–396. [DOI] [PubMed] [Google Scholar]
- 51.Verma R, et al. , Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol, 2018. 3(28). [DOI] [PubMed] [Google Scholar]
- 52.Chai JN, et al. , Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci Immunol, 2017. 2(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KS, et al. , Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science, 2016. 351(6275): p. 858–63. [DOI] [PubMed] [Google Scholar]
- 54.Mucida D, et al. , Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest, 2005. 115(7): p. 1923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawant DV and Vignali DA, Once a Treg, always a Treg? Immunol Rev, 2014. 259(1): p. 173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komatsu N, et al. , Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A, 2009. 106(6): p. 1903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, et al. , Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol, 2007. 37(1): p. 129–38. [DOI] [PubMed] [Google Scholar]
- 58.Bennett CL, et al. , The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet, 2001. 27(1): p. 20–1. [DOI] [PubMed] [Google Scholar]
- 59.Brunkow ME, et al. , Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet, 2001. 27(1): p. 68–73. [DOI] [PubMed] [Google Scholar]
- 60.Mucida D, et al. , Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science, 2007. 317(5835): p. 256–60. [DOI] [PubMed] [Google Scholar]
- 61.Sun CM, et al. , Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med, 2007. 204(8): p. 1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coombes JL, et al. , A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med, 2007. 204(8): p. 1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang SG, et al. , Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. The Journal of Immunology, 2007. 179(6): p. 3724–3733. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Y, et al. , Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature, 2010. 463(7282): p. 808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu L, et al. , Positive and Negative Transcriptional Regulation of the Foxp3 Gene is Mediated by Access and Binding of the Smad3 Protein to Enhancer I. Immunity, 2010. 33(3): p. 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao S, et al. , Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol, 2008. 181(4): p. 2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korn T, et al. , IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A, 2008. 105(47): p. 18460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josefowicz SZ, Lu LF, and Rudensky AY, Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol, 2012. 30: p. 531–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stockinger B, Barthlott T, and Kassiotis G, T cell regulation: a special job or everyone’s responsibility? Nat Immunol, 2001. 2(9): p. 757–8. [DOI] [PubMed] [Google Scholar]
- 70.Round JL and Mazmanian SK, Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A, 2010. 107(27): p. 12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geuking MB, et al. , Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity, 2011. 34(5): p. 794–806. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi S, et al. , Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol, 1995. 155(3): p. 1151–64. [PubMed] [Google Scholar]
- 73.Mottet C, Uhlig HH, and Powrie F, Cutting Edge: Cure of Colitis by CD4+CD25+ Regulatory T Cells. The Journal of Immunology, 2003. 170(8): p. 3939–3943. [DOI] [PubMed] [Google Scholar]
- 74.Broere F, et al. , Oral or Nasal Antigen Induces Regulatory T Cells That Suppress Arthritis and Proliferation of Arthritogenic T Cells in Joint Draining Lymph Nodes. The Journal of Immunology, 2008. 181(2): p. 899–906. [DOI] [PubMed] [Google Scholar]
- 75.Sun JB, et al. , Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3−CD25−CD4+ regulatory T cells. J Immunol, 2006. 177(11): p. 7634–44. [DOI] [PubMed] [Google Scholar]
- 76.Hadis U, et al. , Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity, 2011. 34(2): p. 237–46. [DOI] [PubMed] [Google Scholar]
- 77.Lu L, Barbi J, and Pan F, The regulation of immune tolerance by FOXP3. Nat Rev Immunol, 2017. 17(11): p. 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill JA, et al. , Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity, 2007. 27(5): p. 786–800. [DOI] [PubMed] [Google Scholar]
- 79.Josefowicz SZ, et al. , Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature, 2012. 482(7385): p. 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell C, et al. , Extrathymically Generated Regulatory T Cells Establish a Niche for Intestinal Border-Dwelling Bacteria and Affect Physiologic Metabolite Balance. Immunity, 2018. 48(6): p. 1245–1257.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu W, et al. , A multiple redundant genetic switch locks in the transcriptional signature of T regulatory cells. Nat Immunol, 2012. 13(10): p. 972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang XO, et al. , Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity, 2008. 29(1): p. 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kleinewietfeld M and Hafler DA, The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol, 2013. 25(4): p. 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arroyo Hornero R, et al. , The Impact of Dietary Components on Regulatory T Cells and Disease. Front Immunol, 2020. 11: p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou X, et al. , Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol, 2009. 10(9): p. 1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Komatsu N, et al. , Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med, 2014. 20(1): p. 62–8. [DOI] [PubMed] [Google Scholar]
- 87.Pelly VS, et al. , Interleukin 4 promotes the development of ex-Foxp3 Th2 cells during immunity to intestinal helminths. J Exp Med, 2017. 214(6): p. 1809–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stockis J, Dedobbeleer O, and Lucas S, Role of GARP in the activation of latent TGF-beta1. Mol Biosyst, 2017. 13(10): p. 1925–1935. [DOI] [PubMed] [Google Scholar]
- 89.Hyytiainen M, Penttinen C, and Keski-Oja J, Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci, 2004. 41(3): p. 233–64. [DOI] [PubMed] [Google Scholar]
- 90.Oida T and Weiner HL, TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One, 2010. 5(11): p. e15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersson J, et al. , CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med, 2008. 205(9): p. 1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen ML, et al. , Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol, 2008. 180(11): p. 7327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carrier Y, et al. , Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J Immunol, 2007. 178(1): p. 172–8. [DOI] [PubMed] [Google Scholar]
- 94.Chen ML, et al. , Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol, 2009. 39(12): p. 3423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, et al. , Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science, 1994. 265(5176): p. 1237–40. [DOI] [PubMed] [Google Scholar]
- 96.Miller A, et al. , Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A, 1992. 89(1): p. 421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ochi H, et al. , New immunosuppressive approaches: oral administration of CD3-specific antibody to treat autoimmunity. J Neurol Sci, 2008. 274(1–2): p. 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ochi H, et al. , Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25−LAP+ T cells. Nat Med, 2006. 12(6): p. 627–35. [DOI] [PubMed] [Google Scholar]
- 99.Forster K, et al. , An oral CD3-specific antibody suppresses T-cell-induced colitis and alters cytokine responses to T-cell activation in mice. Gastroenterology, 2012. 143(5): p. 1298–1307. [DOI] [PubMed] [Google Scholar]
- 100.Wu HY, Quintana FJ, and Weiner HL, Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25−LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol, 2008. 181(9): p. 6038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.da Cunha AP, et al. , In vivo anti-LAP mAb enhances IL-17/IFN-gamma responses and abrogates anti-CD3-induced oral tolerance. Int Immunol, 2015. 27(2): p. 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Travis MA, et al. , Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature, 2007. 449(7160): p. 361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edwards JP, Thornton AM, and Shevach EM, Release of active TGF-beta1 from the latent TGF-beta1/GARP complex on T regulatory cells is mediated by integrin beta8. J Immunol, 2014. 193(6): p. 2843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Worthington JJ, et al. , Integrin alphavbeta8-Mediated TGF-beta Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity, 2015. 42(5): p. 903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Travis MA and Sheppard D, TGF-beta activation and function in immunity. Annu Rev Immunol, 2014. 32: p. 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang R, et al. , Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A, 2009. 106(32): p. 13439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stockis J, et al. , Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol, 2009. 39(12): p. 3315–22. [DOI] [PubMed] [Google Scholar]
- 108.Edwards JP, et al. , Regulation of the expression of GARP/latent TGF-beta1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J Immunol, 2013. 190(11): p. 5506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tran DQ, et al. , GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A, 2009. 106(32): p. 13445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuhn C, et al. , IL-6 Inhibits Upregulation of Membrane-Bound TGF-beta 1 on CD4+ T Cells and Blocking IL-6 Enhances Oral Tolerance. J Immunol, 2017. 198(3): p. 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu HY, et al. , In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One, 2011. 6(8): p. e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mayo L, et al. , IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain, 2016. 139(Pt 7): p. 1939–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gagliani N, et al. , Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med, 2013. 19(6): p. 739–46. [DOI] [PubMed] [Google Scholar]
- 114.Roncarolo MG, et al. , Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol, 2014. 380: p. 39–68. [DOI] [PubMed] [Google Scholar]
- 115.Awasthi A, et al. , A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol, 2007. 8(12): p. 1380–9. [DOI] [PubMed] [Google Scholar]
- 116.Levings MK, et al. , Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood, 2005. 105(3): p. 1162–9. [DOI] [PubMed] [Google Scholar]
- 117.Brun V, et al. , Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. Int Immunopharmacol, 2009. 9(5): p. 609–13. [DOI] [PubMed] [Google Scholar]
- 118.Roncarolo MG, et al. , Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews, 2006. 212. [DOI] [PubMed] [Google Scholar]
- 119.Vieira PL, et al. , IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol, 2004. 172(10): p. 5986–93. [DOI] [PubMed] [Google Scholar]
- 120.van Leeuwen MA, et al. , Macrophage-mediated gliadin degradation and concomitant IL-27 production drive IL-10- and IFN-γ-secreting Tr1-like-cell differentiation in a murine model for gluten tolerance. Mucosal Immunology, 2017. 10(3): p. 635–649. [DOI] [PubMed] [Google Scholar]
- 121.Pré MFD, et al. , Tolerance to Ingested Deamidated Gliadin in Mice is Maintained by Splenic, Type 1 Regulatory T Cells. Gastroenterology, 2011. 141(2): p. 610–620.e2. [DOI] [PubMed] [Google Scholar]
- 122.Pot C, et al. , Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol, 2009. 183(2): p. 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Apetoh L, et al. , The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol, 2010. 11(9): p. 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murugaiyan G, et al. , IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol, 2009. 183(4): p. 2435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gregori S, Goudy KS, and Roncarolo MG, The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol, 2012. 3: p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allez M, et al. , Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology, 2002. 123(5): p. 1516–26. [DOI] [PubMed] [Google Scholar]
- 127.Bezie S, et al. , Ex Vivo Expanded Human Non-Cytotoxic CD8(+)CD45RC(low/−) Tregs Efficiently Delay Skin Graft Rejection and GVHD in Humanized Mice. Front Immunol, 2017. 8: p. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vuddamalay Y, et al. , Mouse and human CD8(+) CD28(low) regulatory T lymphocytes differentiate in the thymus. Immunology, 2016. 148(2): p. 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flippe L, et al. , Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol Rev, 2019. 292(1): p. 209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brimnes J, et al. , Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol, 2005. 174(9): p. 5814–22. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Inobe J, and Weiner HL, Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol, 1995. 155(2): p. 910–6. [PubMed] [Google Scholar]
- 132.Girardi M, Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol, 2006. 126(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 133.Vantourout P and Hayday A, Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol, 2013. 13(2): p. 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeong SP, Kang JA, and Park SG, Intestinal intraepithelial TCRgammadelta(+) T cells are activated by normal commensal bacteria. J Microbiol, 2012. 50(5): p. 837–41. [DOI] [PubMed] [Google Scholar]
- 135.Rezende RM, et al. , Identification and characterization of latency-associated peptide-expressing gammadelta T cells. Nat Commun, 2015. 6: p. 8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mengel J, et al. , Anti-gamma delta T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol Lett, 1995. 48(2): p. 97–102. [DOI] [PubMed] [Google Scholar]
- 137.Fujihashi K, et al. , gammadelta T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int Immunol, 1999. 11(12): p. 1907–16. [DOI] [PubMed] [Google Scholar]
- 138.Rezende RM, et al. , gammadelta T Cell-Secreted XCL1 Mediates Anti-CD3-Induced Oral Tolerance. J Immunol, 2019. 203(10): p. 2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katz SI, Parker D, and Turk JL, B-cell suppression of delayed hypersensitivity reactions. Nature, 1974. 251(5475): p. 550–1. [DOI] [PubMed] [Google Scholar]
- 140.Rosser EC and Mauri C, Regulatory B cells: origin, phenotype, and function. Immunity, 2015. 42(4): p. 607–12. [DOI] [PubMed] [Google Scholar]
- 141.Maseda D, et al. , Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol, 2012. 188(3): p. 1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wawrzyniak M, O’Mahony L, and Akdis M, Role of Regulatory Cells in Oral Tolerance. Allergy Asthma Immunol Res, 2017. 9(2): p. 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van de Veen W, et al. , IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol, 2013. 131(4): p. 1204–12. [DOI] [PubMed] [Google Scholar]
- 144.Jones SM, et al. , Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol, 2009. 124(2): p. 292–300, 300.e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Enrique E, et al. , Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol, 2005. 116(5): p. 1073–9. [DOI] [PubMed] [Google Scholar]
- 146.Buchanan AD, et al. , Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol, 2007. 119(1): p. 199–205. [DOI] [PubMed] [Google Scholar]
- 147.Itoh N, Itagaki Y, and Kurihara K, Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Int, 2010. 59(1): p. 43–51. [DOI] [PubMed] [Google Scholar]
- 148.Rachid R and Umetsu DT, Immunological mechanisms for desensitization and tolerance in food allergy. Semin Immunopathol, 2012. 34(5): p. 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karlsson MR, et al. , Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy, 2010. 65(5): p. 561–70. [DOI] [PubMed] [Google Scholar]
- 150.Scadding GW, et al. , Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy, 2010. 40(4): p. 598–606. [DOI] [PubMed] [Google Scholar]
- 151.Kulis M, et al. , Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy, in J Allergy Clin Immunol. 2012: United States. p. 1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim AR, et al. , Mesenteric IL-10-producing CD5+ regulatory B cells suppress cow’s milk casein-induced allergic responses in mice. Sci Rep, 2016. 6: p. 19685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mielle J, et al. , IL-10 Producing B Cells Ability to Induce Regulatory T Cells Is Maintained in Rheumatoid Arthritis. Front Immunol, 2018. 9: p. 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Natarajan P, et al. , Regulatory B cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are CD5 +, express TGF-β, and co-localize with CD4 + Foxp3 + T cells. Mucosal Immunology, 2012. 5(6): p. 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tait Wojno ED and Artis D, Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe, 2012. 12(4): p. 445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stefka AT, et al. , Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A, 2014. 111(36): p. 13145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou L, et al. , Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature, 2019. 568(7752): p. 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sefik E, et al. , Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science, 2015. 349(6251): p. 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang BH, et al. , Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol, 2016. 9(2): p. 444–57. [DOI] [PubMed] [Google Scholar]
- 160.Atarashi K, et al. , Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 2013. 500(7461): p. 232–6. [DOI] [PubMed] [Google Scholar]
- 161.Atarashi K, et al. , Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 2011. 331(6015): p. 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu M, et al. , c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature, 2018. 554(7692): p. 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saraiva M and O’Garra A, The regulation of IL-10 production by immune cells. Nat Rev Immunol, 2010. 10(3): p. 170–81. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y, Su MA, and Wan YY, An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity, 2011. 35(3): p. 337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wohlfert EA, et al. , GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest, 2011. 121(11): p. 4503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Edwards JP, et al. , The GARP/Latent TGF-beta1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance. Eur J Immunol, 2016. 46(6): p. 1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Esterházy D, et al. , Classical dendritic cells are required for dietary antigen-mediated peripheral regulatory T cell and tolerance induction. Nat Immunol, 2016. 17(5): p. 545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Worthington JJ, et al. , Intestinal Dendritic Cells Specialize to Activate Transforming Growth Factor-β and Induce Foxp3+ Regulatory T Cells via Integrin αvβ8, in Gastroenterology. 2011. p. 1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cassani B, et al. , Gut-Tropic T Cells That Express Integrin α4β7 and CCR9 Are Required for Induction of Oral Immune Tolerance in Mice. Gastroenterology, 2011. 141(6): p. 2109–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alameddine J, et al. , Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and IDO-1 Induction. Frontiers in Immunology, 2019. 10: p. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matteoli G, et al. , Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut, 2010. 59(5): p. 595–604. [DOI] [PubMed] [Google Scholar]
- 172.Goubier A, et al. , Plasmacytoid Dendritic Cells Mediate Oral Tolerance. Immunity, 2008. 29(3): p. 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Biswas M, et al. , Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood, 2015. 125(19): p. 2937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Contractor N, et al. , Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol, 2007. 179(5): p. 2690–4. [DOI] [PubMed] [Google Scholar]
- 175.Dasgupta S, et al. , Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe, 2014. 15(4): p. 413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li HS, et al. , Cell-intrinsic role for IFN-alpha-STAT1 signals in regulating murine Peyer patch plasmacytoid dendritic cells and conditioning an inflammatory response. Blood, 2011. 118(14): p. 3879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dubois B, et al. , Sequential Role of Plasmacytoid Dendritic Cells and Regulatory T Cells in Oral Tolerance. Gastroenterology, 2009. 137(3): p. 1019–1028. [DOI] [PubMed] [Google Scholar]
- 178.Perrin GQ, et al. , Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer. Molecular Therapy. Methods & Clinical Development, 2016. 3: p. 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uto T, et al. , Critical role of plasmacytoid dendritic cells in induction of oral tolerance. The Journal of Allergy and Clinical Immunology, 2018. 141(6): p. 2156–2167.e9. [DOI] [PubMed] [Google Scholar]
- 180.Aoki-Yoshida A, et al. , Enhancement of Oral Tolerance Induction in DO11.10 Mice by Lactobacillus gasseri OLL2809 via Increase of Effector Regulatory T Cells. PLOS ONE, 2016. 11(7): p. e0158643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stagg AJ, Intestinal Dendritic Cells in Health and Gut Inflammation. Front Immunol, 2018. 9: p. 2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Waldmann H, et al. , Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev, 2006. 212: p. 301–13. [DOI] [PubMed] [Google Scholar]
- 183.Cobbold SP, et al. , Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A, 2009. 106(29): p. 12055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang Z, et al. , Role of Vitamin A in the Immune System. Journal of Clinical Medicine, 2018. 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu Z-M, et al. , The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cellular and Molecular Immunology, 2015. 12(5): p. 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Medeiros SR, et al. , Vitamin A supplementation leads to increases in regulatory CD4+Foxp3+LAP+ T cells in mice. Nutrition, 2015. 31(10): p. 1260–1265. [DOI] [PubMed] [Google Scholar]
- 187.Zandi-Nejad K, et al. , The role of HCA2 (GPR109A) in regulating macrophage function. The FASEB Journal, 2013. 27(11): p. 4366–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh N, et al. , Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity, 2014. 40(1): p. 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kinoshita M, et al. , Dietary Folic Acid Promotes Survival of Foxp3+ Regulatory T Cells in the Colon. The Journal of Immunology, 2012. 189(6): p. 2869–2878. [DOI] [PubMed] [Google Scholar]
- 190.Abdel-Gadir A, et al. , Microbiota Therapy Acts Via a Regulatory T Cell MyD88/RORγt Pathway to Suppress Food Allergy. Nat Med, 2019. 25(7): p. 1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neumann C, et al. , c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nature Immunology, 2019. 20(4): p. 471–481. [DOI] [PubMed] [Google Scholar]
- 192.Song X, et al. , Microbial bile acid metabolites modulate gut RORγ + regulatory T cell homeostasis. Nature, 2020. 577(7790): p. 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakanishi Y, et al. , Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunology, 2018. 11(2): p. 437–448. [DOI] [PubMed] [Google Scholar]
- 194.Chaudhry A, et al. , CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science (New York, N.Y.), 2009. 326(5955): p. 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huber S, et al. , Th17 Cells Express Interleukin-10 Receptor and Are Controlled by Foxp3− and Foxp3+ Regulatory CD4+ T Cells in an Interleukin-10-Dependent Manner. Immunity, 2011. 34(4): p. 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Waal Malefyt R, et al. , Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. Journal of Experimental Medicine, 1991. 174(4): p. 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rubtsov YP, et al. , Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity, 2008. 28(4): p. 546–58. [DOI] [PubMed] [Google Scholar]
- 198.Murai M, et al. , Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology, 2009. 10(11): p. 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shouval DS, et al. , Interleukin-10 Receptor Signaling in Innate Immune Cells Regulates Mucosal Immune Tolerance and Anti-Inflammatory Macrophage Function. Immunity, 2014. 40(5): p. 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Belghith M, et al. , TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nature Medicine, 2003. 9(9): p. 1202–1208. [DOI] [PubMed] [Google Scholar]
- 201.Collison LW, et al. , The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature, 2007. 450(7169): p. 566–9. [DOI] [PubMed] [Google Scholar]
- 202.Wei X, et al. , Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Reports, 2017. 21(7): p. 1853–1869. [DOI] [PubMed] [Google Scholar]
- 203.Rezende RM and Weiner HL, History and Mechanisms of Oral Tolerance. Seminars in immunology, 2017. 30. [DOI] [PubMed] [Google Scholar]
- 204.Chen Y, et al. , Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci U S A, 1996. 93(1): p. 388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsuji NM, Mizumachi K, and Kurisaki J, Interleukin-10-secreting Peyer’s Patch Cells Are Responsible for Active Suppression in Low-Dose Oral Tolerance. Immunology, 2001. 103(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Santos LM, et al. , Oral Tolerance to Myelin Basic Protein Induces Regulatory TGF-beta-secreting T Cells in Peyer’s Patches of SJL Mice. Cellular immunology, 1994. 157(2). [DOI] [PubMed] [Google Scholar]
- 207.Benson JM, et al. , T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest, 2000. 106(8): p. 1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bedoret D, et al. , Changes in Antigen-Specific T-cell Number and Function During Oral Desensitization in Cow’s Milk Allergy Enabled With Omalizumab. Mucosal immunology, 2012. 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ryan JF, et al. , Successful Immunotherapy Induces Previously Unidentified Allergen-Specific CD4+ T-cell Subsets. Proceedings of the National Academy of Sciences of the United States of America, 2016. 113(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Millington OR, Mowat AM, and Garside P, Induction of bystander suppression by feeding antigen occurs despite normal clonal expansion of the bystander T cell population. J Immunol, 2004. 173(10): p. 6059–64. [DOI] [PubMed] [Google Scholar]
- 211.Turley DM and Miller SD, Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol, 2007. 178(4): p. 2212–20. [DOI] [PubMed] [Google Scholar]
- 212.Navarro S, et al. , Bystander immunotherapy as a strategy to control allergen-driven airway inflammation. Mucosal Immunol, 2015. 8(4): p. 841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kawamoto S, et al. , Foxp3+ T Cells Regulate Immunoglobulin A Selection and Facilitate Diversification of Bacterial Species Responsible for Immune Homeostasis. Immunity, 2014. 41(1): p. 152–165. [DOI] [PubMed] [Google Scholar]
- 214.Mollah ZUA, et al. , Granzyme A Deficiency Breaks Immune Tolerance and Promotes Autoimmune Diabetes Through a Type I Interferon-Dependent Pathway. Diabetes, 2017. 66(12): p. 3041–3050. [DOI] [PubMed] [Google Scholar]
- 215.Velaga S, et al. , Granzyme A Is Required for Regulatory T-Cell Mediated Prevention of Gastrointestinal Graft-versus-Host Disease. PLOS ONE, 2015. 10(4): p. e0124927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bonnet B, et al. , Low-Dose IL-2 Induces Regulatory T Cell-Mediated Control of Experimental Food Allergy. The Journal of Immunology, 2016. 197(1): p. 188–198. [DOI] [PubMed] [Google Scholar]
- 217.Chinen T, et al. , An essential role for the IL-2 receptor in Treg cell function. Nature Immunology, 2016. 17(11): p. 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thornton AM, et al. , Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. Journal of Immunology (Baltimore, Md.: 1950), 2004. 172(11): p. 6519–6523. [DOI] [PubMed] [Google Scholar]
- 219.Valle A, et al. , Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation. J Immunol, 2015. 194(5): p. 2117–27. [DOI] [PubMed] [Google Scholar]
- 220.Kuhn C and Weiner HL, Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy, 2016. 8(8): p. 889–906. [DOI] [PubMed] [Google Scholar]
- 221.Syed A, et al. , Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). Journal of Allergy and Clinical Immunology, 2014. 133(2): p. 500–510.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Varshney P, et al. , A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol, 2011. 127(3): p. 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.AR101 Oral Immunotherapy for Peanut Allergy. New England Journal of Medicine, 2018. 379(21): p. 1991–2001. [DOI] [PubMed] [Google Scholar]
- 224.Di Michele DM, Immune tolerance induction in haemophilia: evidence and the way forward. Journal of thrombosis and haemostasis: JTH, 2011. 9 Suppl 1: p. 216–225. [DOI] [PubMed] [Google Scholar]
- 225.Takedani H, et al. , Ten-year experience of recombinant activated factor VII use in surgical patients with congenital haemophilia with inhibitors or acquired haemophilia in Japan. Haemophilia, 2015. 21(3): p. 374–379. [DOI] [PubMed] [Google Scholar]
- 226.Verma D, et al. , Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci U S A, 2010. 107(15): p. 7101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Alhadj Ali M, et al. , Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med, 2017. 9(402). [DOI] [PubMed] [Google Scholar]
- 228.Walczak A, et al. , Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol, 2013. 70(9): p. 1105–9. [DOI] [PubMed] [Google Scholar]


