Figure 3. Schematic overview of oral tolerance development/oral Treg induction.
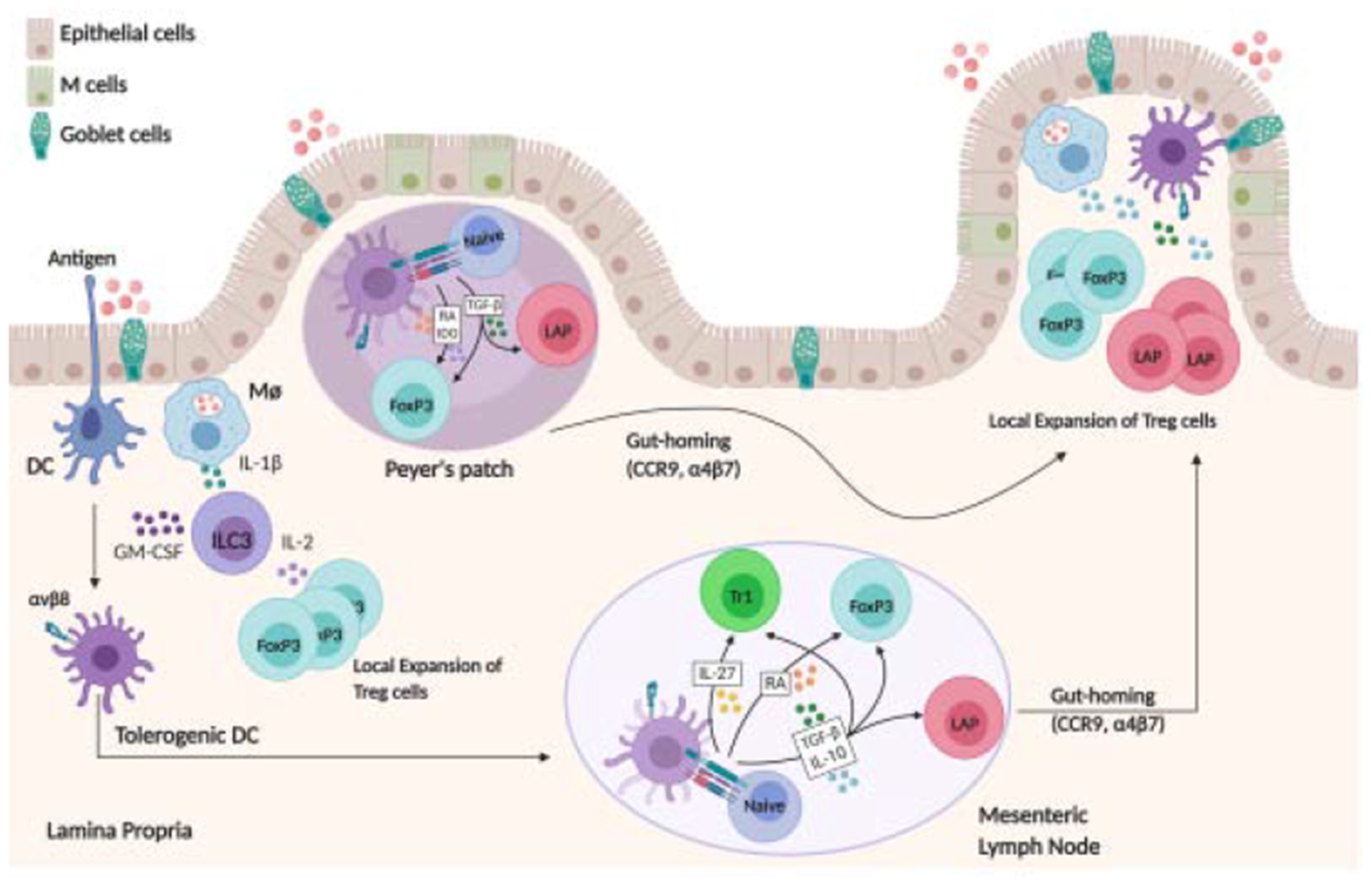
Upon ingestion and the digestive process, antigen reaches the small intestine and can be taken up directly by phagocytes or pass through goblet cell associated passages prior to capture by DC in LP. Microbiota induced IL-1β-secreting intestinal macrophages can induce CD103+ DC differentiation by stimulating GM-CSF-producing ILC3. Moreover, IL-1β-secreting intestinal macrophages induce IL-2 production by ILC3, which in turn stimulates FoxP3+ Treg expansion. Antigen is presented to T cells in the Peyer’s patches or in the MLN through tolerogenic CD103+ DCs, which express the integrin αvβ8 able to activate TGF-β from the latent form and induce Treg. CD103+ DCs produce IDO and RA, which in combination with TGF-β induces FoxP3 Treg differentiation. TGF-β by itself can induce LAP+ Treg differentiation. Tr1 differentiation is triggered by IL-27 producing tolerogenic DCs. CD103+ DCs also mediate upregulation of gut-homing receptors, CCR9 and α4β7 integrin, allowing the recently primed Tregs to home back to the LP, where they undergo local expansion to induce oral tolerance.
