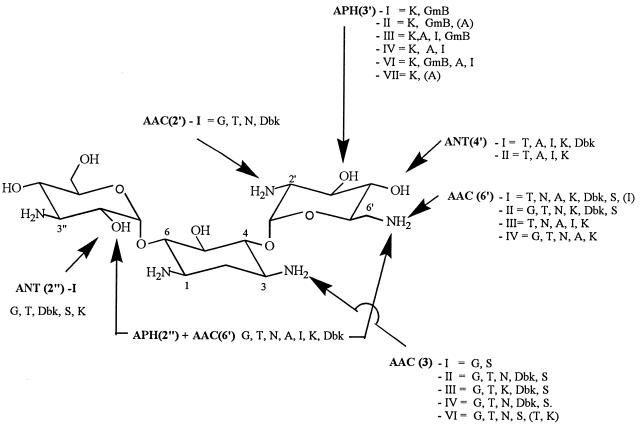
FIG. 3.
Major aminoglycoside-modifying enzymes acting on kanamycin B (this aminoglycoside is susceptible to the largest number of enzymes). Each group of enzymes inactivates specific sites, but each of these sites can be acted upon by distinct isoenzymes (roman numerals) with different substrate specificities (phenotypic classification; each phenotype comprises several distinct gene products [denoted by lowercase letters after the roman numeral in the text]); at least one enzyme is bifunctional and affects both positions 2" (O-phosphorylation) and 6′ (N-acetylation)). The main clinically used aminoglycosides on which these enzymes act are as follows: amikacin (A), dibekacin (Dbk), commercial gentamicin (G) (see text), gentamicin B (GmB), kanamycin A (K), isepamicin (I), netilmicin (N), sisomicin (S), and tobramycin (T) (see text for discussion of arbekacin, sagamicin, and dactimicin). The drug abbreviations which appear in parentheses are those for which resistance was detectable in vitro even though clinical resistance was not conferred. Based on the data of Shaw et al. (89).