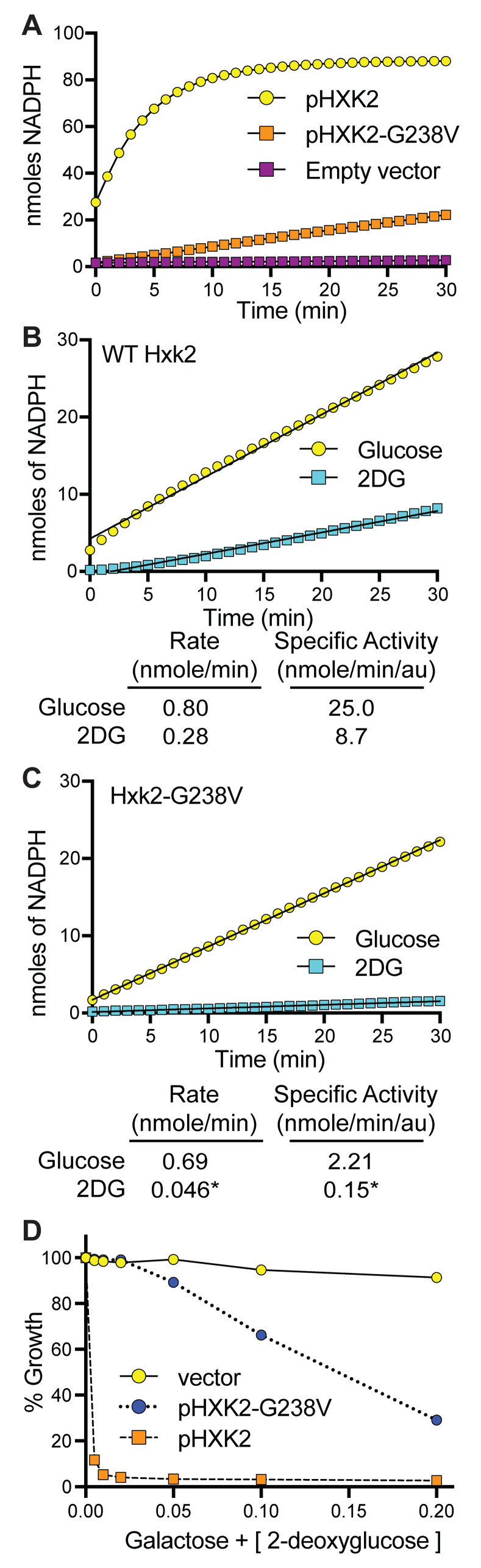
Fig 5. Hxk2G238V has diminished enzymatic activity against glucose and 2DG.
(A) Measure of NADPH production (a proxy for hexose phosphorylation) over time using 5 μg of yeast total protein extracts made from hxk1Δ hxk2Δ glk1Δ cells containing the plasmids indicated. Use of the empty vector serves as a negative control and demonstrates the specificity of this approach for the activity of the plasmid-borne Hxk2. (B-C) Rate of NADPH production in cells expressing WT Hxk2 (B) or Hxk2G238V (C) when total protein extracts were incubated with glucose (yellow) or 2DG (blue). In panel B, 0.68 μg of yeast extract was used (0.032 au per reaction). In panel C, 5.2 μg of yeast extract was used (0.31 au per reaction) to allow the reduced activity of Hxk2G238V to be detectable in our assays. Even with the elevated amount of enzyme used, the rate of NADPH production in Hxk2G238V expressing extracts incubated with 2DG was too low to be accurately measured; the values provided are marked with * to indicate that they are not reliable measures above background. (D) Cells lacking endogenous hexokinase activity (hxk1Δ hxk2Δ glk1Δ) but containing an empty vector or plasmid expressing Hxk2 or Hxk2G238V were grown in galactose and varying concentrations of 2DG. Hxk2G238V-expressing cells are more sensitive to 2DG than those lacking any Hxk2 activity at all, suggesting that Hxk2G238V can phosphorylate 2DG in vivo.