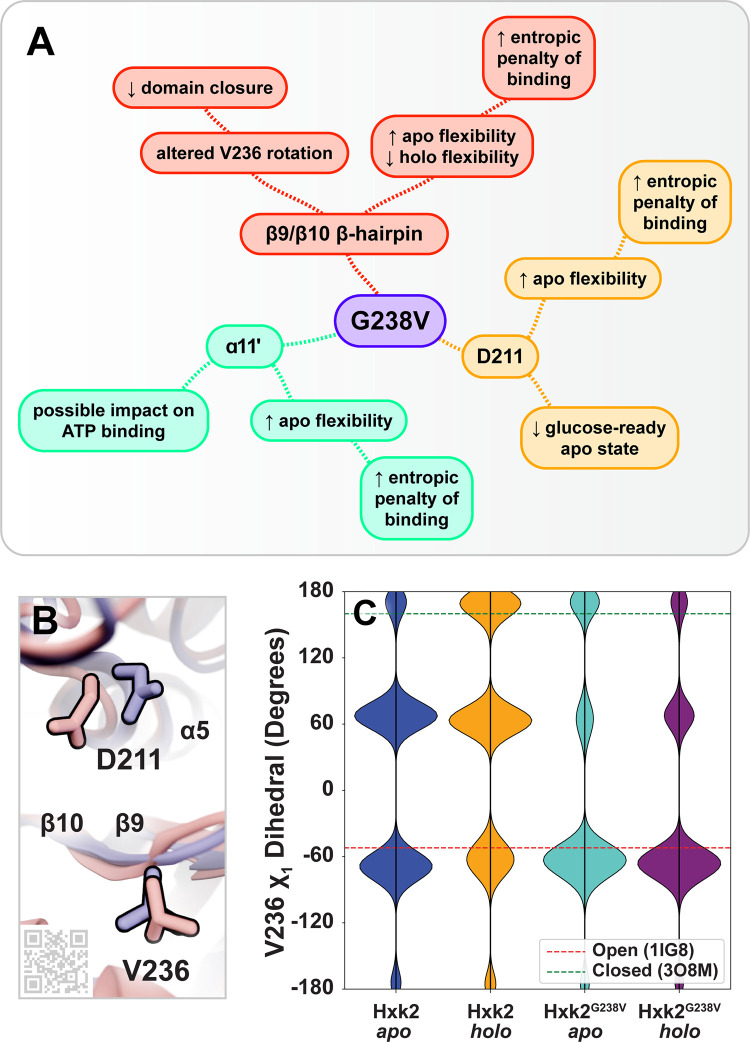
Fig 7. Hypothesized impacts of ScHxk2G238V on the catalytic mechanism.
(A) The G238V mutation alters the dynamics of the β9/β10 β-hairpin, the catalytic D211 residue, and the ɑ11’ helix. The implications of these changes on enzyme function are summarized. (B) Two conformations taken from the ScHxk2G238V apo simulations highlight the movements of V236 (loop residue) and D211 (catalytic residue). In blue, V236 is shown in an open-like conformation, and D211 is shown in a glucose-ready conformation. In pink, V236 is shown in a closed-like conformation, and D211 is shown in a displaced conformation. Key secondary-structure elements are labeled with text, and the substrate-adjacent loop is labeled by its residues (I231-V236). The QR code provides a link to an online ProteinVR scene for virtual-reality visualization. (C) The distributions of the V236 χ1 dihedral angle. For reference, the red and green dashed lines show the corresponding values of the 1IG8 (ScHxk2, open) and 3O8M (KlHxk1, closed) crystal structures, respectively.